1. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003; 21:3798–807.

2. Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004; 22:1103–9.

3. Gupta R, Dastane AM, Forozan F, Riley-Portuguez A, Chung F, Lopategui J, et al. Evaluation of EGFR abnormalities in patients with pulmonary adenocarcinoma: the need to test neoplasms with more than one method. Mod Pathol. 2009; 22:128–33.

4. Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006; 12:839–44.

5. Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010; 2:48–51.
6. Deepak S, Kottapalli K, Rakwal R, Oros G, Rangappa K, Iwahashi H, et al. Real-time PCR: revolutionizing detection and expression analysis of genes. Curr Genomics. 2007; 8:234–51.

7. Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, et al. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2012; 84:1003–11.

8. Guha M, Castellanos-Rizaldos E, Makrigiorgos GM. DISSECT method using PNA-LNA clamp improves detection of EGFR T790m mutation. PLoS One. 2013; 8:e67782.

9. Yao J, Zhang Z, Huang X, Guo Y. Blocker displacement amplification mediated PCR based screen-printed carbon electrode biosensor and lateral flow strip strategy for CYP2C19*2 genotyping. Biosens Bioelectron. 2022; 207:114138.

10. Smith GD, Zhou L, Rowe LR, Jarboe EA, Collins BT, Bentz JS, et al. Allele-specific PCR with competitive probe blocking for sensitive and specific detection of BRAF V600E in thyroid fine-needle aspiration specimens. Acta Cytol. 2011; 55:576–83.
11. Pellestor F, Paulasova P. The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. Eur J Hum Genet. 2004; 12:694–700.

12. Latorra D, Campbell K, Wolter A, Hurley JM. Enhanced allele-specific PCR discrimination in SNP genotyping using 3’ locked nucleic acid (LNA) primers. Hum Mutat. 2003; 22:79–85.

13. Xu J, Pu Y, Lin R, Xiao S, Fu Y, Wang T. PEAC: an ultrasensitive and cost-effective MRD detection system in non-small cell lung cancer using plasma specimen. Front Med (Lausanne). 2022; 9:822200.

14. Thirunavukarasu D, Cheng LY, Song P, Chen SX, Borad MJ, Kwong L, et al. Oncogene concatenated enriched amplicon Nanopore sequencing for rapid, accurate, and affordable somatic mutation detection. Genome Biol. 2021; 22:227.

15. Wang Y, Zhao Y, Bollas A, Wang Y, Au KF. Nanopore sequencing technology, bioinformatics and applications. Nat Biotechnol. 2021; 39:1348–65.

16. Norris AL, Workman RE, Fan Y, Eshleman JR, Timp W. Nanopore sequencing detects structural variants in cancer. Cancer Biol Ther. 2016; 17:246–53.

17. Mimosa ML, Al-Ameri W, Simpson JT, Nakhla M, Boissinot K, Munoz DG, et al. A novel approach to detect IDH point mutations in gliomas using Nanopore sequencing: test validation for the clinical laboratory. J Mol Diagn. 2023; 25:133–42.
18. Pierson-Perry JF, Vaks JE, Vore TE, Durham AP, Fischer C, Gutenbrunner C, et al. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline (EP17-A2). Wayne, PA: Clinical Laboratory Standards Institute;2012.
19. Lopez-Rios F, Angulo B, Gomez B, Mair D, Martinez R, Conde E, et al. Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffinembedded tissue specimens of non-small cell lung cancer. J Clin Pathol. 2013; 66:381–5.

20. Kebschull JM, Zador AM. Sources of PCR-induced distortions in high-throughput sequencing data sets. Nucleic Acids Res. 2015; 43:e143.

21. Malapelle U, Sirera R, Jantus-Lewintre E, Reclusa P, CalabuigFarinas S, Blasco A, et al. Profile of the Roche cobas(R) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev Mol Diagn. 2017; 17:209–15.
22. Wei Z, An T, Wang Z, Chen K, Bai H, Zhu G, et al. Patients harboring epidermal growth factor receptor (EGFR) double mutations had a lower objective response rate than those with a single mutation in non-small cell lung cancer when treated with EGFR-tyrosine kinase inhibitors. Thorac Cancer. 2014; 5:126–32.
23. Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020; 61:167–79.

24. Liang H, Li C, Zhao Y, Zhao S, Huang J, Cai X, et al. Concomitant mutations in EGFR 19Del/L858R mutation and their association with response to EGFR-TKIs in NSCLC patients. Cancer Manag Res. 2020; 12:8653–62.
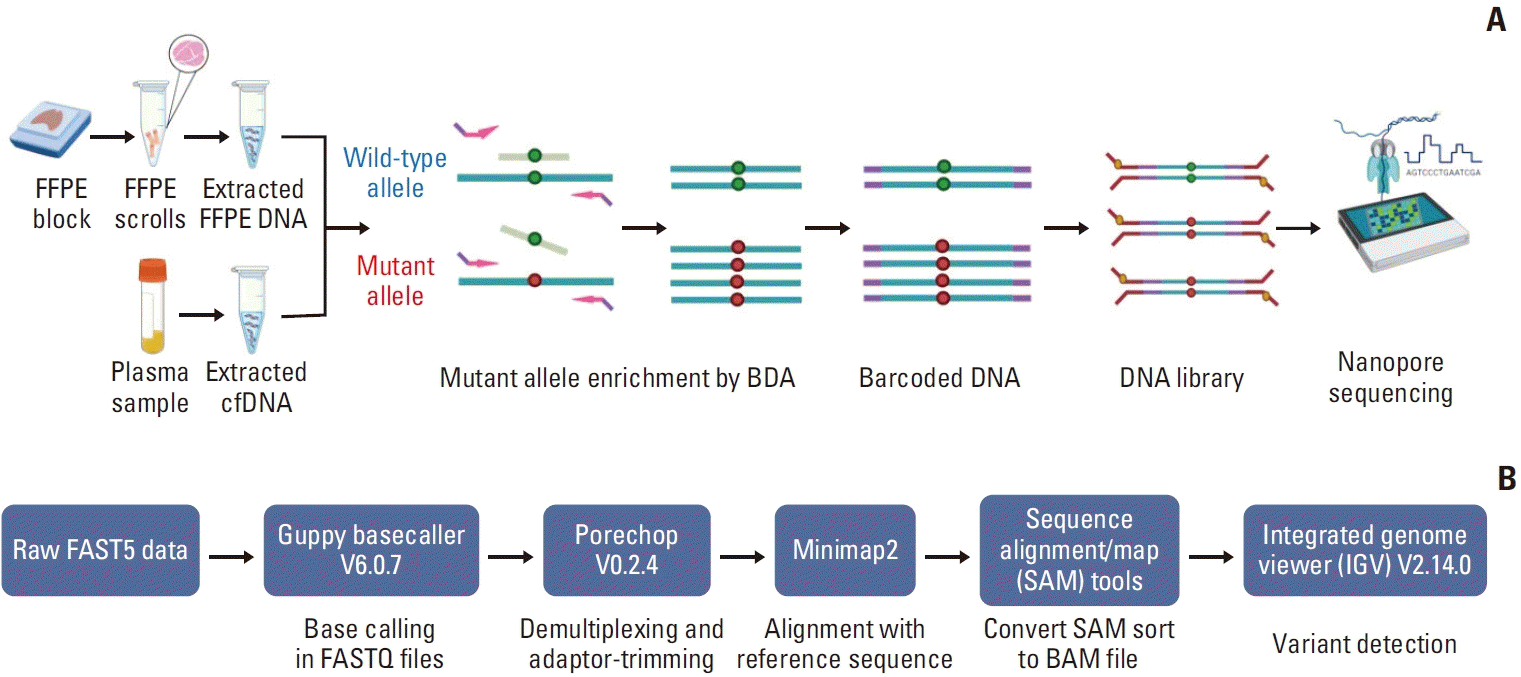
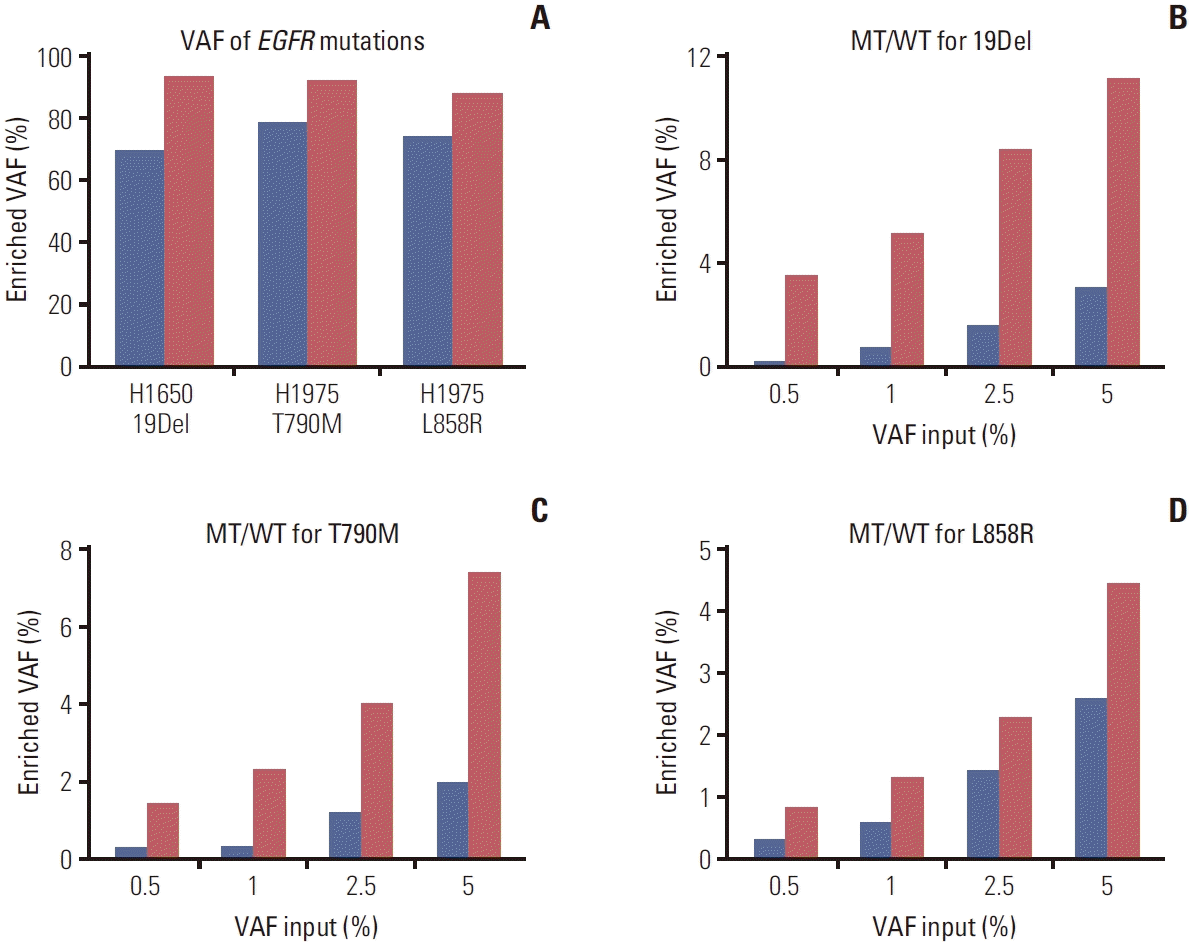
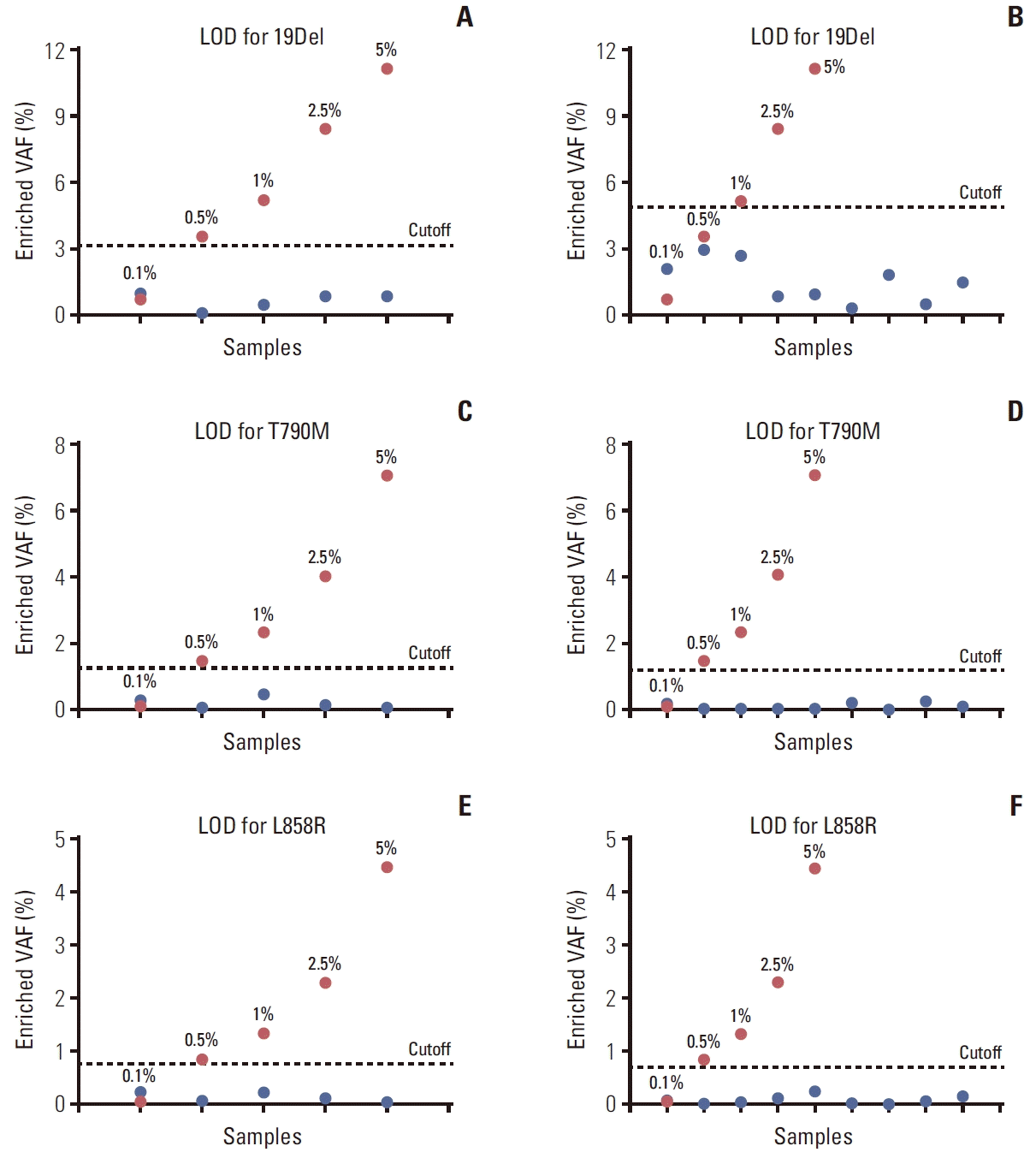




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download