Abstract
Purpose
Methods
Results
Notes
References
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
Supplementary Fig. 1
Supplementary Fig. 2
Supplementary Fig. 3
Supplementary Fig. 4
Fig. 2
Disease-free survival and overall survival of patients according to the M category. M1a group, patients with isolated paraaortic lymph node metastasis; M1bc group, patients with paraaortic lymph node metastasis with other distant metastases.
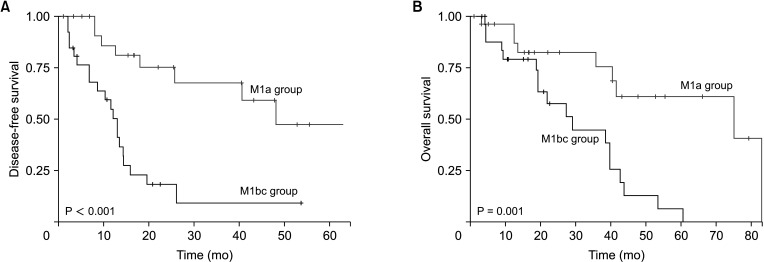
Fig. 3
Disease-free survival and overall survival after paraaortic lymph node dissection according to the number of severity factors of paraaortic lymph node metastasis. Severity factors: number of metastatic paraaortic lymph nodes (mLN) of ≥5, number of harvested paraaortic lymph nodes (hLN) of ≥14, and lymph node ratio (LNR; mLN/hLN) of ≥0.5.

Fig. 4
Disease-free survival and overall survival of the M1a group according to the number of severity factors of paraaortic lymph node metastasis. M1a group, patients with isolated paraaortic lymph node metastasis. Severity factors: number of metastatic paraaortic lymph nodes (mLN) of ≥5, number of harvested paraaortic lymph nodes (hLN) of ≥14, and lymph node ratio (LNR; mLN/hLN) of ≥0.5.

Table 1
Characteristics of colorectal cancer patients with paraaortic lymph node dissection
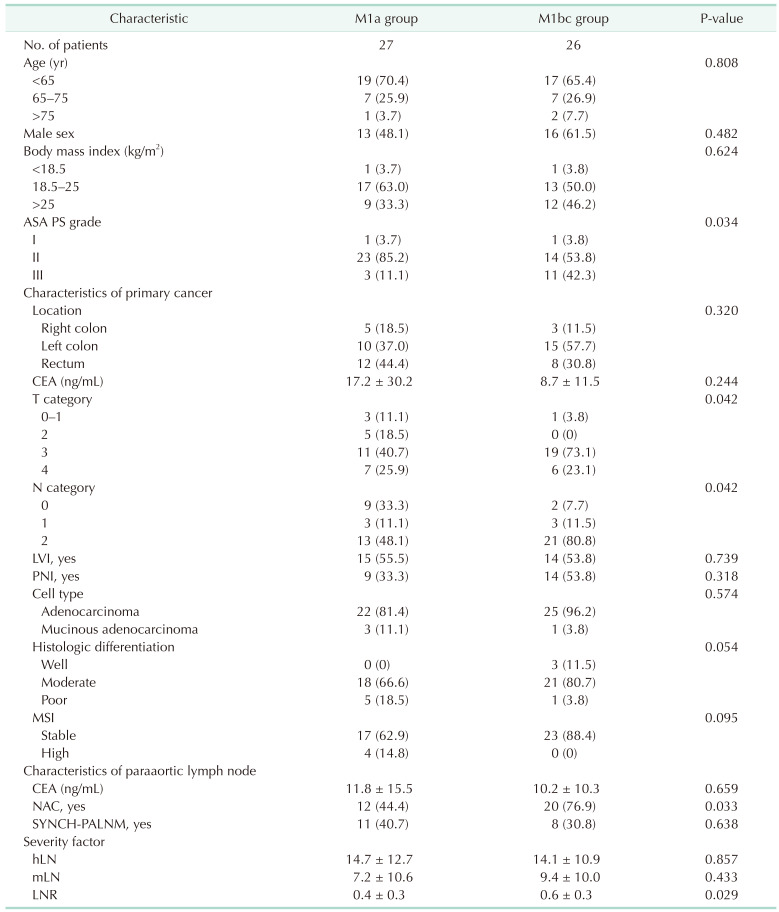
Values are presented as number only, number (%), or mean ± standard deviation.
M1a group, patients with isolated paraaortic lymph node metastasis; M1bc group, patients with metastasis of paraaortic lymph node and other distant organs; ASA PS, American Society of Anesthesiologists physical status; LVI, lymphovascular invasion; PNI, perineural invasion; MSI, microsatellite instability; NAC, neoadjuvant chemotherapy; SYNCH-PALNM, synchronous paraaortic metastasis with primary cancer; hLN, number of harvested paraaortic lymph nodes; mLN, number of metastatic paraaortic lymph nodes; LNR, lymph node ratio (mLN/hLN).
Severity factors were analyzed with only paraaortic lymph nodes, not including regional nodes of primary cancer.
Table 2
Severity factors of paraaortic lymph node metastasis
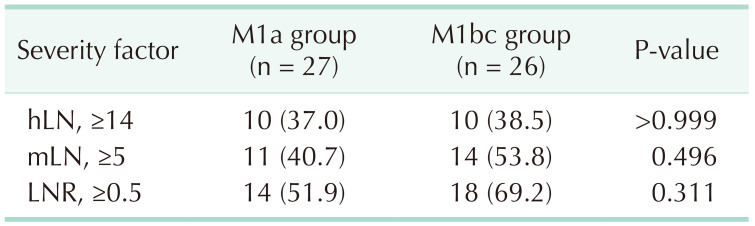
Values are presented as number only, number (%), or mean ± standard deviation.
M1a group, patients with isolated paraaortic lymph node metastasis; M1bc group, patients with metastasis of paraaortic lymph node and other distant organs; hLN, number of harvested paraaortic lymph nodes; mLN, number of metastatic paraaortic lymph nodes; LNR, lymph node ratio (mLN/hLN).
Severity factors were analyzed with only paraaortic lymph nodes, not including regional nodes of primary cancer.
Table 3
Univariate and multivariate analysis for overall survival after paraaortic lymph node dissection
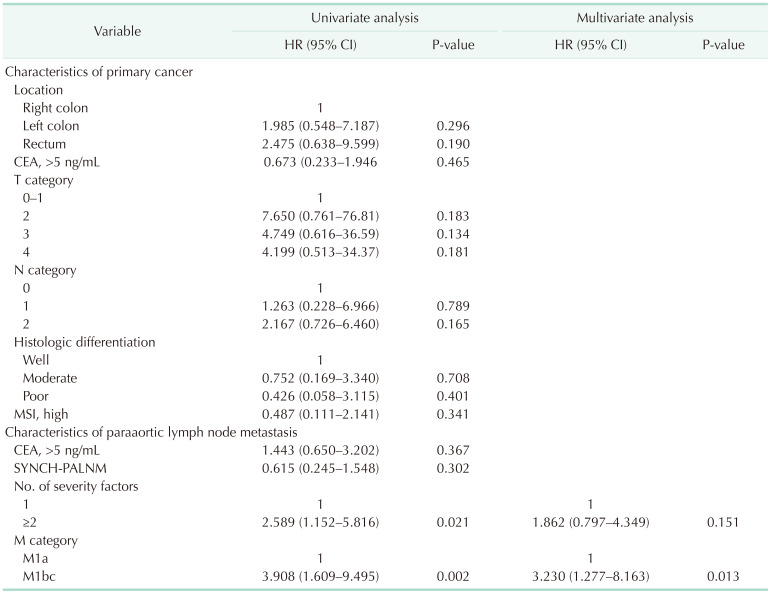
HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; SYNCH-PALNM, synchronous paraaortic lymph node metastasis with primary cancer; M1a, isolated paraaortic lymph node metastasis; M1bc, metastasis of paraaortic lymph node and other distant organs.
Severity factors were analyzed with only paraaortic lymph nodes, not including regional nodes of primary cancer.
Table 4
Univariate and multivariate analysis for disease-free survival after paraaortic lymph node dissection
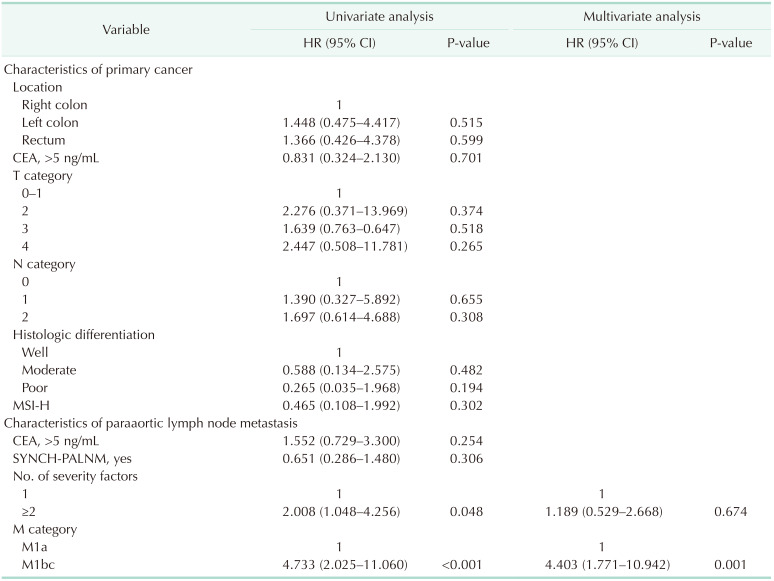
HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; SYNCH-PALNM, synchronous paraaortic lymph node metastasis with primary cancer; M1a, isolated paraaortic lymph node metastasis; M1bc, metastasis of paraaortic lymph node and other distant organs.
Severity factors were analyzed with only paraaortic lymph nodes, not including regional nodes of primary cancer.
Table 5
Multivariate analysis for overall survival and disease-free survival after paraaortic lymph node dissection of the M1a group
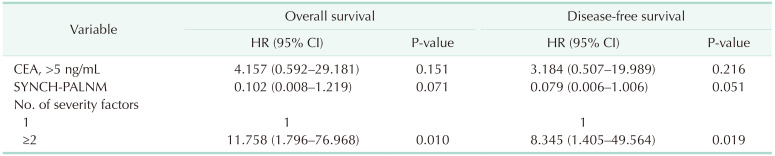
M1a group, patients with isolated paraaortic lymph node metastasis; HR, hazard ratio; CI, confidence interval; SYNCH-PALNM, synchronous paraaortic lymph node metastasis with primary cancer.
Severity factors were analyzed with only paraaortic lymph nodes, not including regional nodes of primary cancer.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download