INTRODUCTION
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, several variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have emerged. Among these variants, omicron (B.1.1.529) was first identified in November 2021 and had been subsequently declared as a variant of concern (VOC) by the World Health Organization (WHO).
1 In South Korea, the first patient carrying the omicron variant was identified on December 1st, 2021,
2 initiating a wide spread of the omicron variant (January 30, 2022 to April 24, 2022),
3 which followed the preceding delta epidemic (July 7, 2021 to January 29, 2021).
Early national and international reports suggested that the clinical course of patients infected with the omicron variant was better than that of patients infected with the delta variant or variants of the other epidemics
4; however, there were also reports suggesting the contrary.
567 The SARS-CoV-2 omicron epidemic in South Korea was massive compared to that in other countries possibly owing to immune evasion
8 or the previous epidemics,
9 and there were reports of many severe patients.
Patients with severe COVID-19 infections require respiratory support, such as a high-flow nasal cannula (HFNC) or mechanical ventilation (MV),
10 and the case fatality rate (CFR) in those patients varies depending on the size and speed of the outbreak. In a previous study, age > 65 years and underlying diseases were found to be risk factors for severe COVID-19 infection and death.
10111213 Studies on young patients, although relatively limited,
141516 reported that not only underlying diseases but also obesity
15 and non-vaccination
171819 had emerged as risk factors of complications in this particular population. A study conducted in South Korea during the fourth epidemic after the delta variant
20 showed that for critical COVID-19 patients aged ≤ 50 years,
21 age, elevated creatinine (Cr), decreased platelet, MV, continuous renal replacement therapy (CRRT), central line-associated bloodstream infection (CLABSI), obesity, and lack of vaccination were risk factors for mortality.
It was suggested that in South Korea, there might be specific differences between the omicron variant epidemic (fifth epidemic) and the previous epidemics with respect to the characteristics and severity of the variant driving the epidemic, epidemic magnitude and spread, and vaccination completion rate at that period. Therefore, this study aimed to analyze the clinical characteristics and risk factors of death in young critical COVID-19 patients in South Korea during the omicron epidemic in comparison to those of patients older than 50 years and to also comparing this period to the delta variant period based on previous data.
DISCUSSION
A previous study
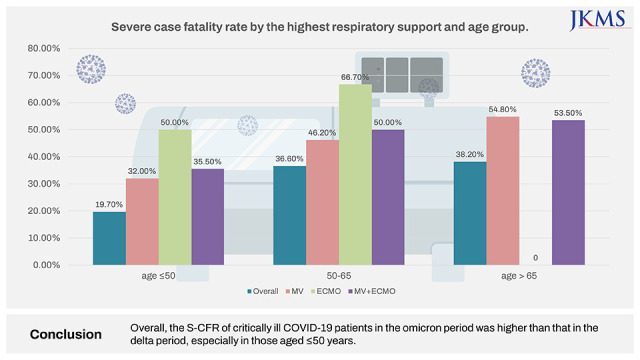
21 conducted in early 2022 analyzed the fatality rate and mortality risk factors in critical COVID-19 patients aged ≤ 50 years who received HFNC or higher respiratory therapy in the delta period, and we attempted to identify the specific characteristics of critical patients aged ≤ 50 years with the same condition in the omicron period. The overall S-CFR reported in the current study was 30.6%, much higher than the overall mortality rate of 1.54% based on the WHO data.
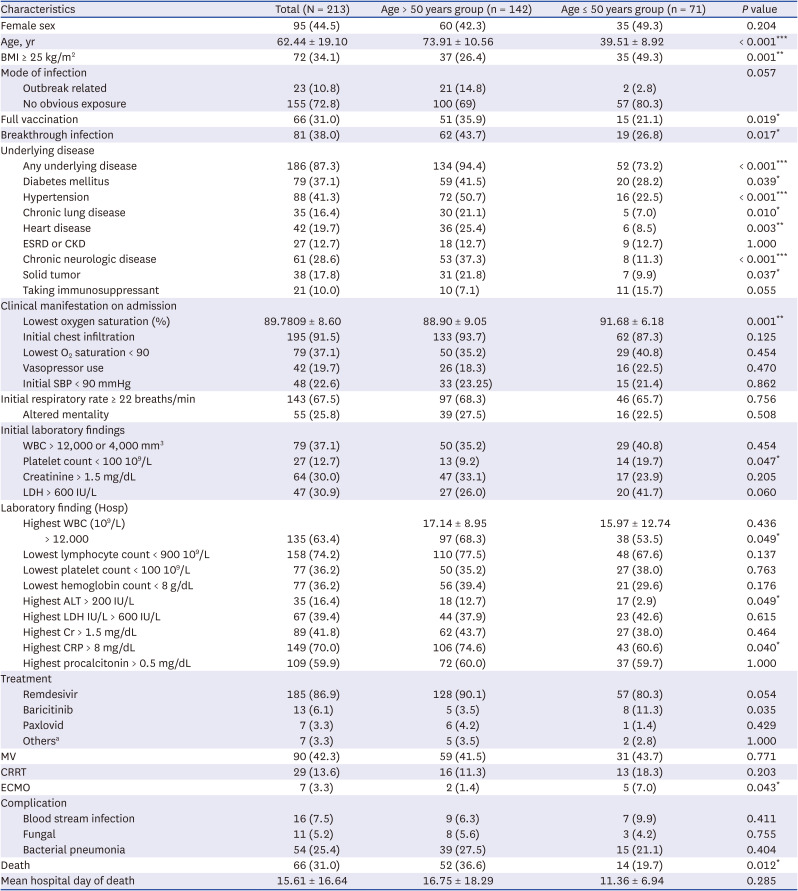
1 Further, it was higher by more than 10% compared to the 21% mortality rate reported by a study conducted during the delta period in patients with similar characteristics and conditions. By age, the CFR for those aged ≤ 50 years increased from 5.6% to 19.7%, which was steeper than that in those aged > 50 years (from 29.3% to 36.6%). The CFR in the current study differs from those in epidemiological reports of the overall COVID-19 omicron pandemic. This is because the CFR analyzed in the current study is the S-CFR, which is the rate of death in severely ill patients. One of the important findings of this study is that unlike the overall population, severely ill patients had high S-CFR, and additional research for other variants are needed.
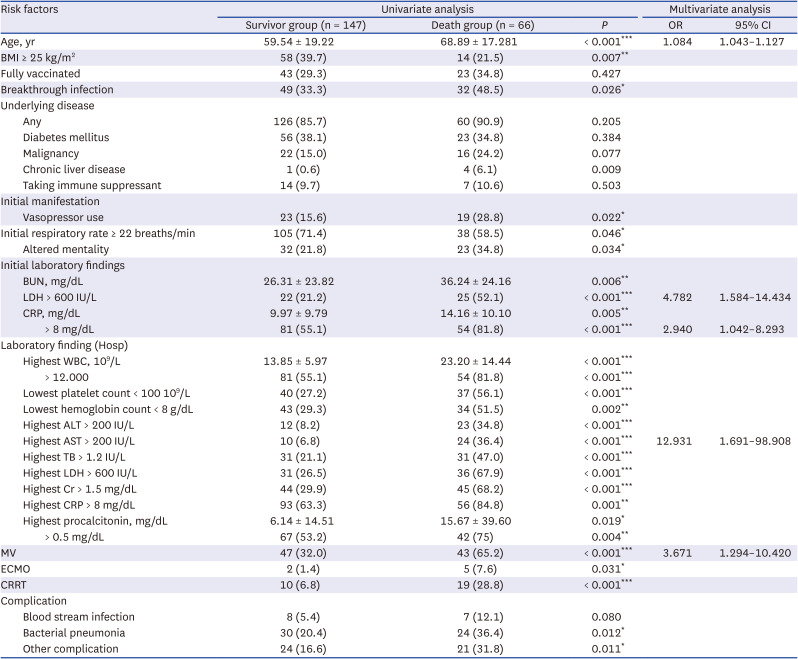
Among the critical patients in the omicron period, in addition to age, initial LDL >600 IU/L, initial CRP > 8 mg/dL, highest AST > 200 IU/L, and MV implementation were significant independent risk factors of mortality, consistent with previous studies.
212324 Meanwhile, BMI, underlying disease, and vaccination status were not significant factors, possibly owing to the higher prevalence of BMI > 25 kg/m
2 and underlying disease in the entire critical patient group than in the general patient population and to the small proportion of fully vaccinated patients.
In a study on adults aged ≥ 65 years in South Korea,
24 HAI, diabetes mellitus, chronic lung disease, chronic neurologic disease, hypoxia, altered mental status, and CRP > 8.0 mg/dL were reported as risk factors for mortality in COVID-19 patients. Unlike data from the delta wave, no significance was observed for underlying diseases in both univariate and multivariate analyses in this study. This result is thought to be because underlying disease is a common risk factor for severity and death. Although the fatality rate was high in the patients with underlying diseases, it did not show any significance as the rate of underlying diseases increased in all critical patients. In contrast to previous findings of underlying diseases such as nosocomial infection
24 and hypertension being a risk factor, the current study found no significance for underlying diseases. This is believed to be possibly owing to the higher frequency of underlying disease among all patients in the omicron period than in the delta period. The current study showed that the frequency of underlying disease in critical patients was significantly higher in the omicron period than in the delta period (87.3% vs. 60.9%,
P < 0.001). The KDCA report
3 showed that 16,118,490 cases were confirmed during the omicron outbreak (fifth period), which corresponded to 95.2% of the cumulative confirmed cases. The daily average number of confirmed cases was 187,424, with a minimum of 17,075 and a maximum of 621,177. This corresponded to 60 times the daily average number of confirmed cases with a total of 649,534 confirmed cases and 3,137.8 daily average confirmed cases during the delta period (fourth period). As mentioned previously, reports have shown that it is difficult to find consistent mortality predictors except for age in large-scale studies in the real world, and the actual prediction rates of several known risk factors are even not high.
2526 Moreover, further studies are needed to explore whether the underlying disease status, age, or the severity of condition at the time of admission predicts morbidity and mortality in a large-scale epidemic. This will be helpful in efficient bed management in the future. With limited resources (hospitals, health care workers), it is crucial to predict the risk of aggravation or mortality of infectious disease because it is a key component of hospital operation.
Although 73.2% of critical patients aged ≤ 50 years during the omicron wave had an underlying disease, Paxlovid and Lagevrio were not prescribed. This was despite their proven effectiveness in preventing exacerbations and their recommendation to be prescribed in patients with COVID-19 with underlying medical diseases. Paxlovid was initially allowed only for limited patients; these included patients aged ≥ 65 years, immunosuppressed patients aged ≥ 12 years, and patients with underlying diseases aged ≥ 50 years or older. After February 21, 2022, the indication for Paxlovid was expanded to senior citizens aged ≥ 60 years, patients with underlying diseases aged ≥ 40 years, and immunocompromised patients aged ≥ 12 years. However, the prescription rate was still very low. This may be proof that there are many cases of late detection in young people who have already progressed to severe disease at the time of diagnosis. Further, it can also be reflection of the low rates of drug prescription for COVID-19 in the general public. The March report of the KDCA
27 showed that Paxlovid, which was introduced on January 13, 2022, was prescribed for only 47,000 cases nationwide over a 2-month period, supporting the above finding. Currently used treatment drugs for COVID-19 have already been proven to be effective.
272829 It is considered that early pharmacological intervention in patients with underlying diseases and risk factors (e.g., obesity), along with an active initial diagnosis, will markedly reduce the severity and mortality related to COVID-19 infection.
A previous study reported that compared to critical patients aged > 50 years, critical patients aged ≤ 50 years had a higher BMI and a lower fatality rate because of a lower severity of their condition at admission. The current study also found that the average BMI of patients in this age group was higher than that of the general population. Further, for patients in this age group, those critical patients during the omicron period had worse S-CFR at the time of hospitalization than those infected in the delta period. Regarding obesity, 49.3% of all critical patients aged ≤ 50 years were obese (BMI ≥ 25 kg/m
2), which is higher than the average in adults. In a study evaluating Korean pediatric COVID-19 patients,
30 8 of the 39,000 pediatric patients had severe disease, and their mean BMI was 29.3 kg/m
2, higher than the national average. Similar trends were observed in our study. Overseas studies
1431 also support the hypothesis that obesity is associated with exacerbation of COVID-19 and mortality. One study explored the mechanism by which obesity is associated with the critical severity of COVID-19
32 and found that the cytokine storm caused by interleukin-6 secretion by adipocytes increased the risk of complications and led to hospitalization and intensive care unit admission. A recent report
33 also showed that 38% of the adult population in 2020 had a BMI ≥ 25 kg/m
2; accordingly, proper COVID-19 management in the increasing obese population should be emphasized.
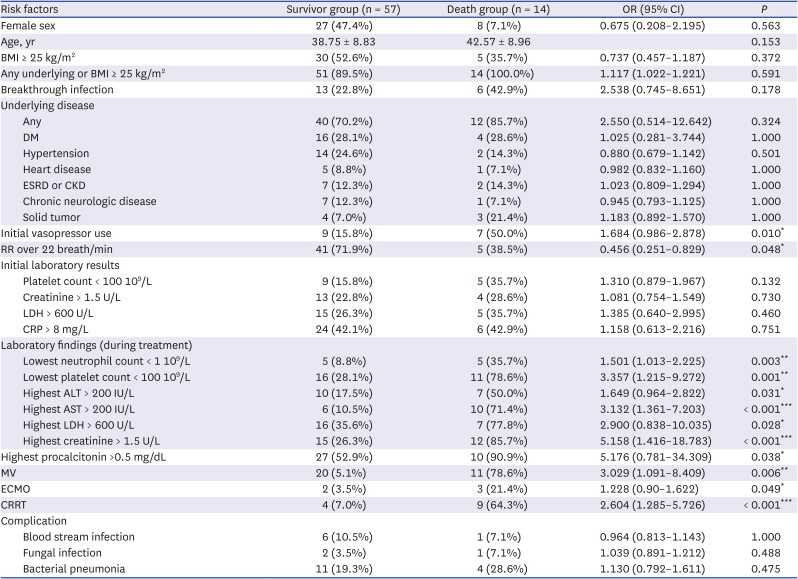
In the current study, among the 14 patients aged ≤ 50 years who died, 5 patients had a BMI of ≥ 25 kg/m
2 (35.7%) (
Supplementary Table 5), which was not significantly different from the average BMI of adults. However, when the underlying diseases were also considered, all of these 14 patients who died had an underlying disease or BMI ≥ 25 kg/m
2. These results can be explained by the relatively low evaluation of BMI in critically ill patients who died as the epidemic increased and the number of critically ill patients increased. Furthermore, some studies abroad have reported that omicron has a lower correlation with BMI than delta,
7 which requires further investigation.
Examining
Tables 1-
3 and
Supplementary Table 1, the laboratory test results or the severity of condition at the time of hospitalization increased significantly in the omicron period from the delta period, and many patients with severe conditions were hospitalized. We hypothesized that this was because of the difference in the epidemic size rather than the effect of the omicron variant itself. As previously mentioned, the total number of confirmed cases during the omicron epidemic was 16,118,490, with an average of 187,424.3 daily confirmed cases, and this was 60 times higher than that during the delta epidemic. The number of beds for severe cases differed only by 500 beds from the maximum estimate of 2,360 beds in the delta period to the maximum estimate of 2,825 (private + public) in the omicron period. As a result, the severity of severe hospitalized patients seems to have differed significantly between the periods. There have been reports that the epidemic during the omicron period was more large scale in South Korea than in other countries because the confirmation rate in previous epidemics was not high.
89 Accordingly, it is thought to have shown different characteristics compared to those of previous epidemics. Hospitals have limited capacity for increasing the number of beds, especially for critically ill patients, owing to limited human resources and facility equipment. Thus, efficient management of severely ill patients using limited beds is more emphasized. In addition, it will be of great help if further studies are conducted for each variant to determine which of the various risk factors are significant in large-scale epidemic situations.
The disease severity, vaccine effectiveness, and mortality rate of the omicron variant infection have not yet been standardized, and findings have been conflicting. Most studies showed that the infection caused by the omicron variant has lower severity and mortality rates than those caused by the delta or alpha variant.
3435 However, there is also evidence that omicron variant has low severity but not mortality.
36 Although some studies reported that for the omicron variant, the probability of critical aggravation was 0.23%,
37 the infectivity was 13 times higher.
38 Moreover, the overall number of critical cases did not seem to decrease significantly, considering the high reinfection rate and immune evasion. In addition, there are still unknown effects of the omicron variant in the real world because there are reports of increasing deterioration of other diseases,
39 in addition to the mortality rate being not low or rather high in specific patient groups,
40 unvaccinated groups,
618 or partially vaccinated groups.
1941 Studies from other countries
540 have also reported that it is difficult to predict the severity, vaccine effectiveness, and mortality rate of the omicron variant infection.
The main reasons why it is challenging to predict the effect of COVID-19 variants like omicron are related to the acquisition of immunity from vaccination and previous infections and the need to analyze different risk factors in specific groups. Studies have shown high mortality related to the omicron variant in certain patient groups,
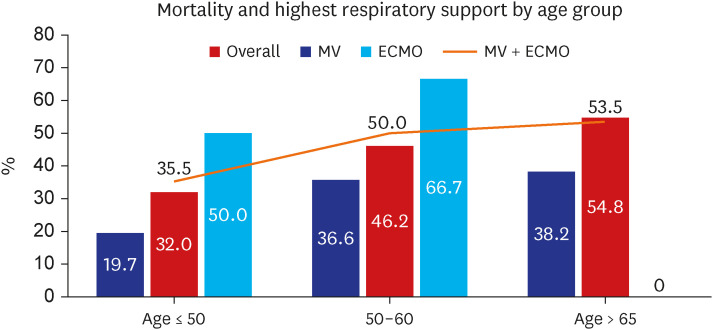
40 including the hospitalized, solid organ transplantation, chronic dialysis, and hematologic malignancy patients. In this study, only Korean critical patients who needed hospitalization were evaluated, and although there was a difference by age, the mortality rate was as high as 19.7% even for those aged 50 years or younger. These results highlight that with the omicron variant, disease severity at the time of diagnosis was worse in certain groups of patients, and further studies should explore this in other critical patients.
Some studies also that showed high mortality in the unvaccinated
618 or partially vaccinated
1941 patients, and consistent results were found in this study. The mortality differs by 3 to 23 times between unvaccinated and vaccinated individuals, but several studies in Europe,
41 South America,
19 Africa,
6 and Asia
18 consistently reported a low mortality rate with the omicron variant owing to the effect of vaccination and immunity. Although the vaccination rate of critical patients during the omicron period was significantly higher than that during the delta period, it was still only 31.0%, which corresponded to less than 50% the national data. In addition, only 15 of the 71 critical patients aged ≤ 50 had completed their vaccination, accounting for only 20%. In particular, only 3 of the 14 patients aged ≤ 50 years who died were vaccinated, showing that the vaccination completion rate was relatively low for all critically ill patients. In this study, which recruited critical patients, although there was no significant difference in the vaccination completion rate between the survivor and death groups, the results highlighted a low vaccination rate in the overall population of critically ill patients.
This study has several limitations. First, there were many cases in which the variant type was not identified, and these cases could not be analyzed according to the variant. However, considering the KCDA report
42 that more than 90% of all patients during the omicron period had omicron and sub-variants, it was thought there would be no significant bias even if all patients were considered to have an omicron variant.
Second, as pointed out in the previous study, we only included severely ill inpatients, which may not be representative of all COVID-19 patients. It is challenging to directly compare the fatality rate before and after the omicron period. Death and survival were compared among critically ill patients, and thus, it was possible that factors found to be significant in other studies (e.g., vaccination and obesity) were found to be insignificant in the current study. While we recognize the limitations of our study design, it is important to note that the title of our study specifically focuses on severely ill patients, which is consistent with the study population we included. Despite these limitations, our study provides important insights into the impact of the Omicron variant on critically ill COVID-19 patients aged ≤ 50 years.
Finally, there may be outcome variables that did not derive a significant outcome value, as there were only 14 deaths among those aged ≤ 50 years. This is believed to be the result of restricting the study population to a specific age range and to specific time periods of the epidemic. Nevertheless, the 14 deaths in patients aged ≤ 50 is significant considering their age and the fact that they are critical patients. We tried to compensate for this by analyzing characteristics of 14 deaths (
Supplementary Table 5). In addition, we compared the delta wave group and the omicron wave group by recruiting the same patient population (i.e., those who had similar characteristics).
Compared to other studies, the current study included a larger number of critical patients aged ≤ 50 years, and data were compared between periods of variant infection. The findings provide important baseline evidence for determining the risk factors of critical disease in the future. Furthermore, because this study was a multicenter study that conducted an additional comparison between epidemic periods, the results have important implications in developing the risk factor criteria for critical patients with COVID-19 omicron infection.
In conclusion, critically ill COVID-19 patients aged ≤ 50 years infected during the omicron period, more than 70% patients have underlying disease. Further, disease severity at the time of hospitalization was worse than that during the delta period owing to the increased epidemic size and the limited number of beds. The effect of obesity on disease severity was relatively low, and the S-CFR reached 20% even for those aged ≤ 50 years. Compared with general population, those with severe infection in the overall population, the age ≤ 50 years group, and the age > 50 years group had significantly lower vaccination rates. All the patients aged ≤ 50 years who died had an underlying disease or had a BMI of ≥ 25 kg/m2. Notably, there was a lack of prescription for Paxlovid for these patients although they satisfied the prescription criteria. Although the severity of the omicron variant epidemic was expected to be relatively low, the total number of critical patients increased as the epidemic size increased. In addition, there was a possibility of progression to critical illness or death in young adult patients infected with the omicron variant. Early diagnosis and active initial treatment was necessary, along with the proven methods of vaccination and personal hygiene. Further studies are needed to explore the difference in mortality among SARS-CoV-2 variants, especially in critical patients.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download