| Korean J Lab Med. 2010 Dec;30(6):567-574. Korean. Published online December 02, 2010. https://doi.org/10.3343/kjlm.2010.30.6.567 | |
| Copyright © 2010 The Korean Society for Laboratory Medicine | |
|
Jeong Tae Kim, M.D.,1
Yong Gon Cho, M.D.,1
Sam Im Choi, M.D.,1
Young Jin Lee, M.D.,2
Hye Ran Kim, Ph.D.,3
Sook Jin Jang, M.D.,4,5
Dae Soo Moon, M.D.,4
Young Jin Park, M.D.,4
and Geon Park, M.D. | |
|
1Department of Laboratory Medicine, Chonbuk National University Medical School, Jeonju, Korea. | |
|
2Department of Laboratory Medicine, Wonkwang University Medical School, Iksan, Korea. | |
|
3Brain Korea 21 Project, Center for Biomedical Human Resource at Chonnam National University, Gwangju, Korea. | |
|
4Department of Laboratory Medicine, Chosun University Medical School, Gwangju, Korea. | |
|
5Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea. | |
| Received April 30, 2010; Revised September 30, 2010; Accepted October 14, 2010. | |
|
Abstract
| |
|
Background
JAK2 genetic variations have been described in a high proportion of patients with BCR/ABL1-negative myeloproliferative neoplasms (MPN). This study was designed to analyze the frequencies of JAK2 V617F and exon 12 variations, and their correlations with clinical characteristics of Korean patients with BCR/ABL1-negative MPN.
Methods
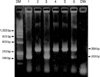
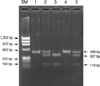
We examined a total of 154 patients with BCR/ABL1-negative MPN that included 24, 26, 89, and 15 patients with polycythemia vera (PV), primary myelofibrosis (PMF), essential thrombocythemia (ET), and unclassified myeloproliferative neoplasms (MPNU), respectively. We performed allele-specific PCR to detect V617F in all BCR/ABL1-negative patients, and performed direct sequencing to detect exon 12 variations in 47 V617F-negative MPN patients. JAK2 c.1641+179_183del5 variation was detected by restriction fragment length polymorphism assay in 176 healthy subjects.
Results
JAK2 V617F was detected in 91 patients (59.1%): PV (91.6%), PMF (46.2%), ET (52.8%), and MPNU (66.7%). In V617F-negative MPN patients, no mutations were found in exon 12. The c.1641+179_183del5 was detected in 68.1% of V617F-negative MPN patients and 45.4% of healthy subjects (P=0.008). JAK2 V617F was closely correlated with age and leukocytosis in BCR/ABL1-negative MPN patients (P<0.05). However, c.1641+179_183del5 was not related to age, sex, or complete blood cell count parameters in V617F-negative MPN patients and healthy subjects. The c.1641+179_183del5 was associated with an increased odds ratio for MPN (odds ratio, 2.6; 95% confidences interval, 1.3-5.1; P=0.007).
Conclusions
Frequencies of V617F are similar to reported results. JAK2 exon 12 mutations may be rare and c.1641+179_183del5 may influence the occurrence of MPN in Korean patients with V6 17F-negative MPN. |
|
Keywords: Myeoloproliferative neoplasms; JAK2; V617F; Exon 12; rs56241661 |
|
|
Figures
|
|
|
|
Tables
|
|
|
|
Notes
|
The present study was supported by grants from the Chosun University (2008).
|
References
|
| 1. | Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008;22:14–22. |
| 2. | Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med 2003;138:819–830. |
| 3. | Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779–1790. |
| 4. | Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle 2007;6:550–566. |
| 5. | Delhommeau F, Jeziorowska D, Marzac C, Casadevall N. Molecular aspects of myeloproliferative neoplasms. Int J Hematol 2010;91:165–173. |
| 6. | Ahn JY, Yoo SJ, Bang SM, Park PW, Seo YH, Shin DB, et al. JAK2V617F mutation in Korean patients with essential thrombocythemia. Korean J Lab Med 2007;27:77–82. |
| 7. | Bang SM, Ahn JY, Park J, Yoo SJ, Park SH, Nam EM, et al. Diagnostic usefulness of the Janus kinase 2 mutation in non BCR/ABL myeloproliferative disorders. Korean J Intern Med 2006;21:219–224. |
| 8. | Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–1061. |
| 9. | Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005;106:2162–2168. |
| 10. | Jones AV, Campbell PJ, Beer PA, Schnittger S, Vannucchi AM, Zoi K, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood 2010;115:4517–4523. |
| 11. | Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group. Semin Thromb Hemost 1997;23:339–347. |
| 12. | Barosi G, Ambrosetti A, Finelli C, Grossi A, Leoni P, Liberato NL, et al. The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol 1999;104:730–737. |
| 13. | Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002;100:2292–2302. |
| 14. | Aplenc R, Orudjev E, Swoyer J, Manke B, Rebbeck T. Differential bone marrow aspirate DNA yields from commercial extraction kits. Leukemia 2002;16:1865–1866. |
| 15. | Saltzman A, Stone M, Franks C, Searfoss G, Munro R, Jaye M, et al. Cloning and characterization of human Jak-2 kinase: high mRNA expression in immune cells and muscle tissue. Biochem Biophys Res Commun 1998;246:627–633. |
| 16. | Quentmeier H, MacLeod RA, Zaborski M, Drexler HG. JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders. Leukemia 2006;20:471–476. |
| 17. | Watanabe S, Arai K. Roles of the JAK-STAT system in signal transduction via cytokine receptors. Curr Opin Genet Dev 1996;6:587–596. |
| 18. | Saharinen P, Takaluoma K, Silvennoinen O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol Cell Biol 2000;20:3387–3395. |
| 19. | Percy MJ, Scott LM, Erber WN, Harrison CN, Reilly JT, Jones FG, et al. The frequency of JAK2 exon 12 mutations in idiopathic erythrocytosis patients with low serum erythropoietin levels. Haematologica 2007;92:1607–1614. |
| 20. | Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–397. |
| 21. | Speletas M, Katodritou E, Daiou C, Mandala E, Papadakis E, Kioumi A, et al. Correlations of JAK2-V617F mutation with clinical and laboratory findings in patients with myeloproliferative disorders. Leuk Res 2007;31:1053–1062. |
| 22. | Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 2007;7:673–683. |
| 23. | Bousquet M, Le Guellec S, Quelen C, Rigal-Huguet F, Delsol G, Brousset P. Frequent detection of the JAK2 V617F mutation in bone marrow core biopsy specimens from chronic myeloproliferative disorders using the TaqMan polymerase chain reaction single nucleotide polymorphism genotyping assay: a retrospective study with pathologic correlations. Hum Pathol 2006;37:1458–1464. |
| 24. | Horn T, Kremer M, Dechow T, Pfeifer WM, Geist B, Perker M, et al. Detection of the activating JAK2 V617F mutation in paraffinembedded trephine bone marrow biopsies of patients with chronic myeoproliferative diseases. J Mol Diagn 2006;8:299–304. |
| 25. | Lay M, Mariappan R, Gotlib J, Dietz L, Sebastian S, Schrijver I, et al. Detection of the JAK2 V617F mutation by LightCycler PCR and probe dissociation analysis. J Mol Diagn 2006;8:330–334. |
| 26. | Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood 2007;110:4030–4036. |
| 27. | Tan AY, Westerman DA, Dobrovic A. A simple, rapid, and sensitive method for the detection of the JAK2 V617F mutation. Am J Clin Pathol 2007;127:977–981. |
| 28. | Pietra D, Li S, Brisci A, Passamonti F, Rumi E, Theocharides A, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood 2008;111:1686–1689. |
| 29. | Schnittger S, Bacher U, Haferlach C, Geer T, Muller P, Mittermuller J, et al. Detection of JAK2 exon 12 mutations in 15 patients with JAK2 V617F negative polycythemia vera. Haematologica 2009;94:414–418. |
| 30. | Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–468. |
| 31. | Butcher CM, Hahn U, To LB, Gecz J, Wilkins EJ, Scott HS, et al. Two novel JAK2 exon 12 mutations in JAK2 V617F-negative polycythaemia vera patients. Leukemia 2008;22:870–873. |
| 32. | NCBI Single Nucleotide Polymorphism. [Updated on Mar 2010].
http://www.ncbi.nlm.nih.gov/projects/SNP/snp_
|
| 33. | Pardanani A, Lasho TL, Finke CM, Gangat N, Wolanskyj AP, Hanson CA, et al. The JAK2 46/1 haplotype confers susceptibility to essential thrombocythemia regardless of JAK2V617F mutational status-clinical correlates in a study of 226 consecutive patients. Leukemia 2010;24:110–114. |




 ePub
ePub Citation
Citation Print
Print