Abstract
Polycythemia vera (PV) was first described nearly 120 years ago. In the subsequent century, the clinical syndrome of PV, its natural history, its treatment, and many critical pathogenetic features of the disease were characterized. The discovery of the Janus-associated kinase - 2 mutation JAK2 V617F and the characterization of its role in myeloproliferative neoplasms have substantially changed the diagnostic paradigm for PV, and have potential to lead to new therapy and new pathogenetic insights.
Go to : 
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by an absolute erythrocytosis not driven by erythropoietin (Epo), i.e., a primary polycythemia. It is distinguished from other primary polycythemias by the involvement of other hematopoietic cell lineages [1]. Like other myeloproliferative neoplasms, PV is associated with increased risks of evolution to leukemia and of thrombosis [2]; like other polycythemia syndromes, many of its signs and symptoms result from increased blood viscosity [1].
Credit for the first description of PV is generally given to Henri Vaquez, who in 1892 reported a patient with a polycythemia syndrome not due to cardiopulmonary disease [3]. He later proposed that erythrocytosis could be divided into two syndromes: absolute erythrocytosis or polycythemia, resulting from an increased red cell mass; and relative erythrocytosis, resulting from a reduction in plasma volume without increased red cell mass [4]. In 1903, William Osler recognized that several polycythemia cases reported in the literature or encountered in his extensive consulting practice represented a single clinical syndrome not previously delineated, and published a description of this syndrome [5]. The Viennese physician Wilhelm Türk subsequently recognized that PV was not solely as disease of the erythron but involved other hematopoietic lineages [6], and the basic clinical features of PV were largely defined by the time Osler wrote a second, shorter review of PV in 1908 [7]. The history of the early definition of PV has been reviewed in more detail elsewhere [8].
Go to : 
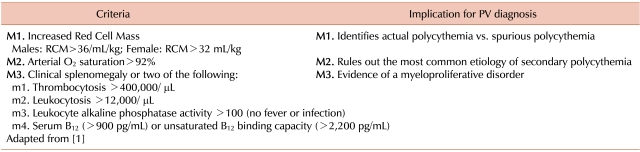
From its initial descriptions until the last few years, the diagnosis of PV has been made by criteria that were based upon clinical and clinical laboratory findings. In 1966, the U.S. National Cancer Institute funded the Polycythemia Vera Study Group (PVSG) to carry out randomized prospective trials in PV with the goal of defining the best treatment modality [9]. One of the requirements for developing a credible clinical trials program was the establishment of specific criteria for the diagnosis of PV. The subsequent PVSG diagnostic criteria (Table 1) were the basis for all PV diagnostic schema for 40 years [10].
The PVSG criteria essentially reflect the need to establish three features to make the diagnosis of PV. First, the patient must have actual polycythemia and not relative polycythemia (M1). Second, it must be shown that the patient does not have secondary polycythemia (most commonly due to hypoxemia) (M2). Finally, evidence of a myeloproliferative neoplasm needed to be demonstrated (M3 or two minor criteria). The PVSG study criteria are based on clinical or clinical laboratory parameters, other than the requirement for demonstration of an increased red cell mass by nuclear medicine methods. Pathologic parameters (such as bone marrow morphology) were not part of the system, and of course molecular diagnostic parameters were not available in 1966.
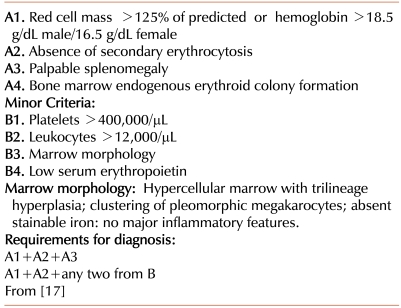
Subsequent developments in research on the pathogenesis of PV have been incorporated into the basic PVSC criteria structure as alternatives or supplements to an original criterion. For example, studies of the coefficient of variation in red cell mass studies has led to the proposal that a red cell mass greater than 125% of predicted value for the local laboratory is a more accurate definition than the specific red cell mass values included in the original criteria [11]. The development of reliable and clinically applicable assays for Epo has led to these assays being suggested as a more conclusive method to rule out secondary polycythemia [12, 13]. Alternative major or minor criteria for evidence of a myeloproliferative neoplasm include splenomegaly only apparent on radiologic studies [11], demonstration of erythroid colony formation in vitro without added Epo (endogenous erythroid colonies, or EEC) [14], increased expression of the platelet receptor Mpl [15], or of gene PRV-1 [16]. However, these alternatives, as expressed in the 2001 World Health Organization (WHO) criteria [17] (Table 2), preserved the basic structure and underlying assumptions of the PVSG criteria.
Go to : 
In 2005, Kralovics and colleagues reported the results of an analysis of the Janus-associated kinase 2 (JAK2) gene on chromosome 9 in 244 patients with myeloproliferative neoplasms (128 with PV) [18]. They observed a dominant gain-of-function mutation in which the valine at position 617 was replaced by phenylalanine (V617F) in approximately half the patients. This mutation appeared to confer an in vitro proliferative advantage upon cells into which it was transfected [18].
Over the next five years, studies of JAK2 V617F experienced proliferation as well. Mutations of JAK2 V617F were reported to occur in 80-96% of cases of PV, and to correlate strongly with the 2001 WHO criteria [19-21]. Studies of PV patients lacking JAK2 V617F reported that a significant number of these had mutations in exon 12 of the JAK2 gene resulting in a myeloproliferative phenotype, although there was some suggestion that exon 12 mutations were more likely to be associated with a picture of pure erythrocytosis [22-26]. Unlike the BCR/ABL mutation in chronic myelogenous leukemia, JAK2 V617F is not pathognomonic for PV but is seen in both essential thrombocytosis (ET) and myelofibrosis [18]. It has been reported that JAK2 V617F expression identifies a subset of ET patients who are predisposed to thrombosis and evolution to myelofibrosis (i.e., more like PV patients) [27, 28]. The allele burden of JAK2 V617F mutations has also been proposed as a tool to distinguish ET (low allele burden) from PV (high allele burden) [29]. The JAK2 V617F allele burden is also associated with response to hydroxyurea, with a high allele burden predicting responsiveness [30], and a reduction in allele burden correlating with clinical response to hydroxyurea [31].
In addition to its role in clinical PV, the identification of JAK2 V617F has had implications for the understanding of the pathogenesis of PV. Transplantation of mice with JAK2 V617F expressing cells leads to a myeloproliferative neoplasm resembling PV [32, 33]. JAK2 V617F appears to be required for the characteristic Epo hypersensitivity of PV erythroid progenitors [34]. However, acquisition of JAK2 mutations does not appear to be the initial molecular event in the pathogenesis of PV, but is rather downstream from some as yet undetermined initiating event [35, 36].
Go to : 
As noted above, JAK2 mutation status can serve as a predictor of response to treatment with hydroxyurea [30], and changes in JAK2 V617F status are associated with response to either hydroxyurea or interferon [31, 37]. Reports of agents targeted to JAK2 in PV are largely limited to studies using various PV model systems [38-40] or to clinical trials in post-PV myelofibrosis [41, 42]. The variable results found in the reported clinical studies may be either agent-specific or specific to the patient subset selected for the study.
Go to : 
As discussed earlier, JAK2 V617F is not pathognomonic of PV but rather is a hallmark of BCR/ABL-negative myeloproliferative neoplasms. Therefore, if it was to be incorporated in a diagnostic schematic analogous to the PVSG criteria, it would be an alternative to M3, which indicates a myeloproliferative neoplasm.
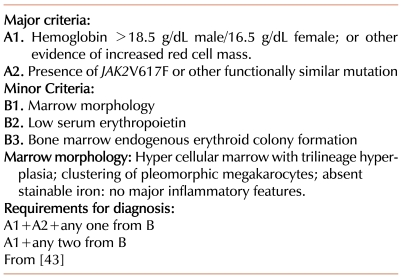
However, the 2008 modification of the WHO diagnostic criteria for PV [43] (Table 3) departed substantially from the underlying concepts of the PVSG criteria. The first major criterion (A1) remains the demonstration of an increased red cell mass, but employs a widely, though not universally [44], accepted criterion based on hemoglobin concentration. The diagnosis of PV is then made by the finding of two additional criteria. This requirement can be met by demonstrating any two of the following: a low serum Epo concentration, a characteristic bone marrow morphology, the presence of EEC, or JAK2 V617F (or a functionally similar mutation, like JAK2 exon 12). This departs substantially from a reliance on readily available clinical and clinical laboratory parameters to more complex testing. PV has become less a clinical diagnosis and more a pathologic and molecular diagnosis.
Go to : 
JAK2 testing has been suggested as the initial step in the evaluation of erythrocytosis [45]; however, there is no study describing how the general population of hematologists uses this test which could be compared to the report by Strieff et al. in 2002, which describes the diagnostic and management practices prevalent in the early post-PVSG era [46]. In a recent report, the use of JAK2 V617F in a single academic practice was described [47]. Patients newly presenting with a diagnosis of PV were routinely tested for the mutation. Of established patients, patients who did not meet the 2001 WHO diagnostic criteria fully tended to be more likely to be tested for JAK2 V617F mutation; patients who seemed to have a clearly established diagnosis.
Go to : 
The discovery of the JAK2 V617F mutation, the definition of its frequency in PV and other myeloproliferative neoplasms, and the identification of its pathophysiologic implications, have transformed the diagnostic approach to PV from one based in the clinic and clinical laboratory to a truly molecular paradigm. JAK2 V617F has the potential to guide therapy of PV and other BCR/ABL-negative myeloproliferative neoplasms, whether as a therapeutic target or as a marker of response to therapy or of progression risk.
Go to : 
Notes
Presented in part as the 2010 Hematology/Oncology State of the Art Lecture, Southern Society of Clinical Investigation, New Orleans LA USA, February 27, 2010.
Go to : 
References
1. Means RT. Greer JP, Foerster J, Rodgers GM, editors. Polycythemia vera. Wintrobe's Clinical Hematology. 2009. Baltimore, USA: Lippincott Williams & Wilkins;p. 2031–2044.
2. Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995; 123:656–664. PMID: 7574220.
3. Vaquez H. Sur une forme speciale de cyanose s'accompanant d'hyperglobulie excessive et persistante. CR Soc Biol (Paris). 1892; 4:384.
4. Vaquez H, Quiserne P. Des polyglobulies. CR Soc Biol (Paris). 1902; 12:915.
5. Osler W. Chronic cyanosis with polycythemia and enlarged spleen: a new clinical entity. Am J Med Sci. 1903; 126:187–201.
6. Türk W. Beiträge zur Kenntnis des symptomenbildes Polyzythämie mit Milztumor und Zyanose. Wien Med Wochenschr. 1904; 17:153–160.
7. Osler W. Erythremia (polycythemia with cyanosis, Maladie de Vaquez). Lancet. 1908; 1:143–146.
8. Means RT Jr. Perspective: Osler's 1903 paper on polycythemia vera. Am J Med Sci. 2008; 335:418–419. PMID: 18552569.

9. Berlin NI, Wasserman LR. Polycythemia vera: a retrospective and reprise. J Lab Clin Med. 1997; 130:365–373. PMID: 9358074.

10. Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986; 23:132–143. PMID: 3704665.
11. Pearson TC. Evaluation of diagnostic criteria in polycythemia vera. Semin Hematol. 2001; 38(1 Suppl 2):21–24. PMID: 11242598.

12. Cotes PM, Doré CJ, Yin JA, et al. Determination of serum immunoreactive erythropoietin in the investigation of erythrocytosis. N Engl J Med. 1986; 315:283–287. PMID: 3724821.

13. Birgegård G, Wide L. Serum erythropoietin in the diagnosis of polycythaemia and after phlebotomy treatment. Br J Haematol. 1992; 81:603–606. PMID: 1390249.

14. Casadevall N, Vainchenker W, Lacombe C, et al. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982; 59:447–451. PMID: 7055650.

15. Moliterno AR, Spivak JL. Posttranslational processing of the thrombopoietin receptor is impaired in polycythemia vera. Blood. 1999; 94:2555–2561. PMID: 10515857.

16. Bock O, Serinsöz E, Neusch M, Schlué J, Kreipe H. The polycythaemia rubra vera-1 gene is constitutively expressed by bone marrow cells and does not discriminate polycythaemia vera from reactive and other chronic myeloproliferative disorders. Br J Haematol. 2003; 123:472–474. PMID: 14617008.

17. Michiels JJ, De Raeve H, Berneman Z, et al. The 2001 World Health Organization and updated European clinical and pathological criteria for the diagnosis, classification, and staging of the Philadelphia chromosome-negative chronic myeloproliferative disorders. Semin Thromb Hemost. 2006; 32(4 Pt 2):307–340. PMID: 16810609.

18. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–1790. PMID: 15858187.
19. Ganly P, Hanrahan V, Baker B, Romeril K. Identification of JAK2V617F in patients with polycythemia is highly correlated with conventional criteria for diagnosis of polycythemia vera. Am J Hematol. 2007; 82:80–82. PMID: 16924638.
20. Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005; 106:2162–2168. PMID: 15920007.

21. Verstovsek S, Silver RT, Cross NC, Tefferi A. JAK2V617F mutational frequency in polycythemia vera: 100%, >90%, less? Leukemia. 2006; 20:2067. PMID: 16990780.
22. Scott LM, Beer PA, Bench AJ, Erber WN, Green AR. Prevalance of JAK2 V617F and exon 12 mutations in polycythaemia vera. Br J Haematol. 2007; 139:511–512. PMID: 17910642.

23. Bernardi M, Ruggeri M, Albiero E, Madeo D, Rodeghiero F. Isolated erythrocytosis in V617F negative patients with JAK2 exon 12 mutations: report of a new mutation. Am J Hematol. 2009; 84:258–260. PMID: 19229983.

24. Kouroupi E, Zoi K, Parquet N, et al. Mutations in exon 12 of JAK2 are mainly found in JAK2 V617F-negative polycythaemia vera patients. Br J Haematol. 2008; 142:676–679. PMID: 18503583.

25. Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi A. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2 V617F-negative polycythemia vera. Leukemia. 2007; 21:1960–1963. PMID: 17597810.
26. Pietra D, Li S, Brisci A, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood. 2008; 111:1686–1689. PMID: 17984312.

27. Cheung B, Radia D, Pantelidis P, Yadegarfar G, Harrison C. The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythaemia. Br J Haematol. 2006; 132:244–245. PMID: 16398659.

28. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005; 131:208–213. PMID: 16197451.
29. Hussein K, Bock O, Theophile K, et al. JAK2(V617F) allele burden discriminates essential thrombocythemia from a subset of prefibrotic-stage primary myelofibrosis. Exp Hematol. 2009; 37:1186–1193. PMID: 19616600.

30. Sirhan S, Lasho TL, Hanson CA, Mesa RA, Pardanani A, Tefferi A. The presence of JAK2V617F in primary myelofibrosis or its allele burden in polycythemia vera predicts chemosensitivity to hydroxyurea. Am J Hematol. 2008; 83:363–365. PMID: 18266209.
31. Girodon F, Schaeffer C, Cleyrat C, et al. Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxyurea therapy. Haematologica. 2008; 93:1723–1727. PMID: 18728027.

32. Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008; 111:5109–5117. PMID: 18334677.

33. Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006; 66:11156–11165. PMID: 17145859.

34. Dupont S, Massé A, James C, et al. The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007; 110:1013–1021. PMID: 17389763.
35. Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006; 108:1377–1380. PMID: 16675710.

36. Nussenzveig RH, Swierczek SI, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007; 35:32–38. PMID: 17198871.

37. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008; 112:3065–3072. PMID: 18650451.

38. Pardanani A, Hood J, Lasho T, et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007; 21:1658–1668. PMID: 17541402.

39. Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009; 23:1441–1445. PMID: 19295546.

40. Gozgit JM, Bebernitz G, Patil P, et al. Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL-xL survival signaling in the human JAK2 V617F cell line SET-2. J Biol Chem. 2008; 283:32334–32343. PMID: 18775810.

41. Santos FP, Kantarjian HM, Jain N, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010; 115:1131–1136. PMID: 20008298.

42. Hitoshi Y, Lin N, Payan DG, Markovtsov V. The current status and the future of JAK2 inhibitors for the treatment of myeloproliferative diseases. Int J Hematol. 2010; 91:189–200. PMID: 20191331.

43. Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007; 110:1092–1097. PMID: 17488875.

44. Johansson PL, Safai-Kutti S, Kutti J. An elevated venous haemoglobin concentration cannot be used as a surrogate marker for absolute erythrocytosis: a study of patients with polycythaemia vera and apparent polycythaemia. Br J Haematol. 2005; 129:701–705. PMID: 15916693.

45. James C, Delhommeau F, Marzac C, et al. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia. 2006; 20:350–353. PMID: 16341032.

46. Streiff MB, Smith B, Spivak JL. The diagnosis and management of polycythemia vera in the era since the Polycythemia Vera Study Group: a survey of American Society of Hematology members' practice patterns. Blood. 2002; 99:1144–1149. PMID: 11830459.

47. Means RT Jr. JAK2 V617F mutation testing in polycythemia vera: use and impact in an academic practice. Am J Med Sci. 2008; 336:327–329. PMID: 18854675.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download