| Korean J Gastroenterol. 2015 Dec;66(6):350-353. English. Published online December 22, 2015. https://doi.org/10.4166/kjg.2015.66.6.350 | |
| Copyright © 2015 The Korean Society of Gastroenterology | |
|
Ji Young Lee,
In Tae Moon,
Hye Young Lee,
Hang Lak Lee
and Dong Soo Han | |
| Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. | |
| Received June 12, 2015; Revised August 15, 2015; Accepted August 16, 2015. | |
|
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by- | |
|
Abstract
| |
|
Lower gastrointestinal complications often develop in end stage renal disease patients, and among the more problematic is recurrent colon ulcer. The exact pathogenesis of this condition is not known and there were no specific therapeutic modalities concerning this type of disease entity. We report, with a literature review, a case of recurrent colon ulcer with intermittent hematochezia in an end stage renal disease patient on long term hemodialysis that improved after conversion to peritoneal dialysis. |
|
Keywords: Chronic kidney failure; Gastrointestinal hemorrhage; Colon; Ulcer; Renal dialysis |
|
|
INTRODUCTION
|
Recurrent colon ulcer is a rare condition of unknown etiology that is easily overlooked. It is known by many different names including bleeding cecal ulcer,1 simple ulcer of colon, discrete colon ulcer and idiopathic colon ulcer. In end stage renal disease, many lower gastrointestinal complications including ischemic bowel disease, spontaneous colonic perforation, fecal impaction, abscess and fistula of the bowel, strictures, adhesions, small bowel obstruction, diverticulitis with perforation, appendicitis, and peritonitis have been noted2 and colon ulcer occurs in end stage renal disease patients. However, the exact pathogenesis of this condition is not known and there were no specific therapeutic modalities concerning this type of disease entity.
We report here, with a literature review, a case of recurrent colon ulcer with intermittent hematochezia in an end stage renal disease patient on long-term hemodialysis that improved after conversion to peritoneal dialysis.
|
CASE REPORT
|
A 54-year-old man was admitted to our hospital with a moderate amount of hematochezia and constant, vague, diffuse lower abdominal pain. His medical history included old pulmonary tuberculosis, hypertension, diabetes mellitus, brain infarction, and end-stage renal disease due to diabetic nephropathy, for which he was on hemodialysis thrice weekly for seven months. His current medications were aspirin, ticlopidine, furosemide, atenolol and angiotensin converting enzyme inhibitor. His colonoscopic findings from ten months prior were nonspecific. He reported no symptoms such as nausea, vomiting, constipation, diarrhea, weight loss, and melena. He appeared chronically ill with vital signs as follows body temperature 36.5℃, heart rate 90 beats/min, respiratory rate 14 times/min and blood pressure 150/90 mmHg. Physical examination revealed a soft abdomen with tenderness on left lower quadrant, and normal bowel sounds. On rectal examination, there were blood clots, but no hemorrhoid and no palpable masses on abdomen and no hepatosplenomegaly. No blood was evident in nasogastric aspiration of stomach. Cardiac and chest examination were unremarkable.
Laboratory examination revealed a white blood cell count of 7,900/mm3, differential white blood cell counts within normal limits, hemoglobin 8.2 g/dL, hematocrit 23.9%, platelet count 109,000/mm3, BUN 54 mg/dL, creatinine 9.4 mg/dL. Electrolytes, urine analysis, coagulation factor and other blood chemistry values were within normal limits. Stool examination and cultures for enteric pathogens were also negative. Serum cytomegalovirus (CMV) antigen test and CMV antibody test were negative. Serum CEA level was within normal range.
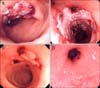
Colonoscopy was performed one day after initial hematochezia, revealing an acute single hemorrhagic ulceration of 3 cm in diameter with blood clots at 25 cm from the anal verge and surrounding normal mucosa. There was no abnormal finding from anus to terminal ileum except that one (Fig. 1A). We injected 1:10,000 epinephrine to control bleeding. Six biopsy specimens from the lesion revealed a piece of necrotic ulcer detritus and colonic mucosa with chronic inflammation, no granulomas and no inclusion bodies (Fig. 2). The patient had no further bleeding and his symptoms improved. We empirically used 5-aminosalicylic acid orally and restarted aspirin and ticlopidine. A colonoscopy two months later revealed a healed colon ulcer. Since the last colonoscopy, the patient continued to have small amounts of intermittent hematochezia for 10 months. One year later, he was readmitted with a moderate amount of hematochezia. Sigmoidoscopy revealed a web-like material composed of a huge blood clot and mucus aggregation with ulceration at 25 cm from the anal verge. The mucosa surrounding the ulcer was normal (Fig. 1B). We stopped aspirin and ticlopidine again. A colonoscopy seven days later, from anus to terminal ileum, showed a large map-like ulcer on the mesenteric side 25 cm from anal verge and exposed vessels on the surface of the ulcer. Five biopsy specimens from the lesion revealed necrotic ulcer detritus and colonic mucosa with chronic inflammation. There was no evidence of Crohn's disease, CMV colitis and tuberculosis. Abdominal computed tomography scan showed diffuse bowel wall thickening on the long segment of proximal sigmoid colon with surrounding fat infiltration and normal mesenteric vasculature. We performed an echocardiogram to evaluate heart function; the result was normal. Two months later, hematochezia developed again. Colonoscopy showed reduction in size of the previous ulcer, but newly developed ulcers with blood clots were noted in the proximal portion (Fig. 1C). Medication such as aspirin and ticlopidine could cause colon ulceration, but it recurred even after cessation of these medications (Fig. 3). Therefore, we ruled out medication-induced colon ulceration. We suggest that occlusive or nonocclusive mesenteric ischemia is an important etiology for nonspecific colon ulcer in this patient. We converted dialysis modality from hemodialysis to peritoneal dialysis. Three months later, colonoscopy revealed healed colonic ulcers with bowel wall contraction around the ulcer site (Fig. 1D). He is now visiting the outpatient department regularly, and there has been no hematochezia for four years since discharge.
|
|
|
|
DISCUSSION
|
Colon ulcers are rare and occasionally can be life-threatening.3 The possible etiologies of colon ulcer are very diverse, but can be broadly characterized as vascular, medication, or mechanical trauma. Vascular causes include vasculitis,4 atherosclerosis,5 and atheromatous microembolization6; medication causes include NSAIDs,7 oral contraceptives,8 and cortisone5; mechanical trauma causes include intussusception9 and surgical trauma.10 Diverticulitis,11 radiation,5 burns,12 bacteria,13 and viruses13 are also possible etiologies. However, any vascular etiology is difficult to confirm. There are several reports of nonspecific ulcer of the colon in chronic hemodialysis patients.14 Colon ulceration has been reported as a complication of renal transplantation or transplant nephrectomy.1, 15 The majority of the reported isolated colon ulcers in transplant patients have been attributed to viruses, either CMV or varicella.1 However it seems that localized bowel ischemic change in chronic hemodialysis patient can also be a cause. Thrombosed blood vessels are sometimes seen in the submucosa.16 Hardie and Nicoll17 described three cases of idiopathic colon ulcer. All three showed microvascular fibrin thrombi with overlying infarct necrosis and ulceration. In a histologic analysis of 26 cases of nonspecific ulcer, seven of the 26 cases had atherosclerosis and three had thrombosis.5 We suspect that bowel perfusion defect induced by continuous thrombus formation and frequent hemodynamic change in hemodialysis patients is a cause of nonspecific colon ulcer.18 Hemodialysis is capable of inducing subclinical bowel ischemia, and this phenomenon is primarily related to ultrafiltration and hemodynamic instability. However, since the ultrafiltration rate is low in peritoneal dialysis, we switched to peritoneal dialysis from hemodialysis.19
After conversion to peritoneal dialysis, the colon ulcer healed and there was no further recurrence of colon ulcer. Although we did not perform a mesenteric angiogram, we think that it may show a thrombosis or significant atherosclerosis. The relationship of colon ulceration to end-stage renal disease is obscure. However, there have been some reports of colon ulceration in chronic hemodialysis patients,14 so chronic hemodialysis may pose a risk of colon ulceration.
In conclusion, although there are many possible causes of nonspecific colon ulcer, vascular origin such as atherosclerosis and vascular thrombosis should be suspected especially in hemodialysis patient. Therefore, management reducing ischemic bowel damage may be required in such cases, and we think that conversion to peritoneal dialysis is an appropriate management plan. We reported the first case of successful management of recurrent colon ulcer in a hemodialysis patient after conversion to peritoneal dialysis. We think that our case suggests a new management modality of nonspecific colon ulcer and adds to the understanding of the pathogenesis of nonspecific colon ulcer in hemodialysis patients.
|
Notes
|
Financial support:None.
Conflict of interest:None.
|
References
|
| 1. | Sutherland DE, Chan FY, Fourcar E, Simmons PL, Howard RJ, Najarian JS. The bleeding cecal ulcer in transplant patients. Surgery 1979;86:386–398. |
| 2. | Adams PL, Rutsky EA, Rostand SG, Han SY. Lower gastrointestinal tract dysfunction in patients receiving long-term hemodialysis. Arch Intern Med 1982;142:303–306. |
| 3. | Losanoff JE, Richman BW, Foerst JR, Griesemer AD, Mundis GM, Jones JW. Nonspecific ulcers of the colon. Endoscopy 2003;35:521–525. |
| 4. | Lee RG. The colitis of Behçet's syndrome. Am J Surg Pathol 1986;10:888–893. |
| 5. | Rubio CA, Nydahl S. "Nonspecific" erosions and ulcers of the colonic mucosa. Dig Dis Sci 1994;39:821–826. |
| 6. | Pfeifer M, Kogel H, Heymer B. Cholesterol crystal embolization, a rare cause of postoperative ischemic colitis. Vasa 1990;19:54–58. |
| 7. | Robinson MH, Wheatley T, Leach IH. Nonsteroidal antiinflammatory drug-induced colonic stricture. An unusual cause of large bowel obstruction and perforation. Dig Dis Sci 1995;40:315–319. |
| 8. | Bernardino ME, Lawson TL. Discrete colonic ulcers associated with oral contraceptives. Am J Dig Dis 1976;21:503–506. |
| 9. | Miller LS, Barbarevech C, Friedman LS. Less frequent causes of lower gastrointestinal bleeding. Gastroenterol Clin North Am 1994;23:21–52. |
| 10. | Ando Y, Kikuchi K, Ichikawa N, et al. A nonspecific colonic ulcer occurring after hepatectomy: report of a case. Surg Today 1997;27:353–356. |
| 11. | Fisher JK. Intramural diverticulosis simulating aphthous ulceration of the colon. Mo Med 1986;83:687–689. |
| 12. | Still JM Jr, Scheirer RC, Law EJ. Caecal perforation due to colonic ulcer in a burn patient. Burns 1994;20:85–86. |
| 13. | Koçdor MA, Aydin C, Astarcioğlu H, Küpelioğlu A, Bora S. Perforated solitary ulcer of the colon. Report of a case. Dis Colon Rectum 1998;41:1059–1061. |
| 14. | Mills B, Zuckerman G, Sicard G. Discrete colon ulcers as a cause of lower gastrointestinal bleeding and perforation in end-stage renal disease. Surgery 1981;89:548–552. |
| 15. | Margolis DM, Etheredge EE, Garza-Garza R, Hruska K, Anderson CB. Ischemic bowel disease following bilateral nephrectomy or renal transplant. Surgery 1977;82:667–673. |
| 16. | Margaretten W, McKay DG. Thrombotic ulcerations of the gastrointestinal tract. Arch Intern Med 1971;127:250–253. |
| 17. | Hardie IR, Nicoll P. Localized ulceration of the caecum due to microcirculatory thrombosis. A new concept of non-specific ulceration of the caecum. Aust N Z J Surg 1973;43:149–157. |
| 18. | Song JH, Kim JI, Jung JH, et al. A case of phlebosclerotic colitis in a hemodialysis patient. Korean J Gastroenterol 2012;59:40–43. |
| 19. | McIntyre CW. Hemodynamic effects of peritoneal dialysis. Perit Dial Int 2011;31 Suppl 2:S73–S76. |




 ePub
ePub Citation
Citation Print
Print