Abstract
Elevated lactate levels are associated with acute illnesses, and the mortality is high. Here, we report a case of lactate-containing peritoneal dialysis (PD) solution inducing lactic acidosis corrected by changing to hemodialysis (HD). This 70-year-old female patient was treated with PD 8 months previously for end-stage renal disease caused by diabetes mellitus. She was admitted complaining of general weakness. Initial lactate level was 22.1 mg/dL and increased to 62.4 mg/dL showing high anion gap metabolic acidosis and compensatory hyperventilation. There are no definite causes of lactic acidosis besides the use of PD solutions containing a lactate component. The patient's lactate level was decreased after temporarily changing the dialysis modality to HD. Her lactate level was increased again after restarting PD, and decreased to normal after restarting HD. We report this case because physicians should consider lactate-containing PD solution as a possible cause of lactic acidosis.
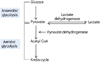
Generally, lactate is removed by the liver and kidney. Pyruvate is produced via glycolysis and then gets in the Krebs cycle in aerobic conditions, while lactate is an end product of glycolysis and supplies into the Cori cycle in anaerobic conditions (Fig. 1).1 Elevated serum lactate levels are related to increased lactate production and decreased lactate clearance.2 The causes of lactic acidosis are divided into conditions associated with tissue hypoxia (type A, hypovolemic, septic or cardiogenic shock, bowel ischemia) and disorders without tissue hypoxia (type B, seizure, diabetes, thiamine deficiency, malignancy, liver failure, toxins and medication).3 Decreased pyruvate dehydrogenase activity, caused by hypoxia and thiamine deficiency, and increased lactate dehydrogenase (LDH) are related to hyperlactatemia. Metformin is a well-known case of lactic acidosis, especially in patients with renal dysfunction.4
Previous reports have demonstrated that severe lactic acidosis can be treated with bicarbonate-containing peritoneal dialysis (PD) solution.5 Recent PD solutions contain bicarbonate and lactate or only lactate. Here, we report case of lactate-containing PD solution inducing lactic acidosis corrected by changing the treatment modality to hemodialysis (HD). Lactic acidosis is defined as a blood lactate level higher than normal range (4.5-19.8 mg/dL) with high anion gap metabolic acidosis (lower than pH 7.35). This 70-year-old female patient was treated with PD 8 months ago for end-stage renal disease (ESRD) caused by diabetes mellitus (DM). There was no possible cause for her lactic acidosis. We report this case because physicians should consider lactate-containing PD solutions as a possible cause of lactic acidosis.
On May 2013, a 70-year-old woman who received PD 8 months ago for ESRD caused by DM was admitted with severe general weakness. Four PD solutions were used each day: two 1.5% PhysionealTM PD solutions, one 4.25% PhysionealTM PD solution, and one overnight exchange using 7.5% ExtranealTM PD solution (Baxter Health care Corporation).
Her DM was controlled with insulin. She had a history of brain infarction in 2001 and mitral valve replacement due to mitral valve regurgitation in 2002. She began to take levetiracetam (Keppra®), an anti-epileptic agent, due to seizures in 2009. She continued to take this until recently.
Initial investigations revealed the following levels: creatinine 6.7 mg/dL, LDH 753 IU/L, and lactate 22.1 mg/dL. Arterial blood gas analysis yielded the following levels: pH 7.436, PaCO2 38.7 mmHg, PaO2 81.0 mmHg, bicarbonate 25.5 mEq/L, and anion gap (AG) 15.5. On hospital day 4, laboratory studies revealed the following levels: lactate 33.0 mg/dL, pH 7.468, PaCO2 30.6 mmHg, PaO2 99.2 mmHg, bicarbonate 21.7 mEq/L, and AG 20.3. On hospital day 15, the respiratory rate was increased to 32 and the lactate level was elevated to 62.4 mg/dL. Her blood gas showed severe high AG metabolic acidosis with compensatory respiratory alkalosis and the following levels: pH 7.479, PaCO2 19.2 mm Hg, PaO2 117.0 mmHg, bicarbonate 13.9 mEq/L, and AG 24.1 (Table 1). There were no definite causes of the patient's lactic acidosis. We checked pyruvate and thiamine levels after lactic acidosis was occurred. Pyruvate level was 2.7 mg/dL (normal range: 0.3-0.9 mg/dL), lactate-to-pyruvate ratio was 10.5, and thiamine level was 125.4 nmol/L (normal range: 59-213 nmol/L).
We hypothesized that lactate-containing PD solution was one of the causes of her lactic acidosis. We decided to temporarily change the mode of renal replacement therapy (RRT) to HD. The following day, significant improvement of acid-base status was observed, and the lactate level was de creased to 32.1 mg/dL. On the 8th day of HD treatment, PD was restarted because the lactate level had been decreased to 13.3 mg/dL. However, the lactate level was increased again to 32.1 mg/dL and compensatory hyperventilation reoccurred. Inevitably, PD was stopped and HD was restarted. The patient was discharged 34 days later with a lactate level of 12.8 mg/dL. During the follow-up period, the patient's lactate level remained normal (Fig. 2).
PD is an important RRT for ESRD patients worldwide, and is used in approximately 7-8% of the total dialysis population. PD solution is composed of a buffer, an electrolyte, an osmotic agent and so on.6 Bicarbonate was the first buffer used in PD solutions to help correct acidosis. However, because bicarbonate and calcium may precipitate during storage, lactate is used as a buffer instead. Lactate-based fluids are acidic (approximately pH 5.5), and may exhibit cytotoxic effects in vitro. After the advent of multi-chambered PD delivery systems, it became possible to replace lactate with bicarbonate. Now, bicarbonate/lactate-based and lactate-based solutions are available.
Physioneal™ PD solutions use a mixture of buffers including 18.648 mmol/L of lactate and 18.648 mmol/L of bicarbonate. Extraneal™ PD solutions use a buffer with 49.728 mmol/L of lactate only. Although lactate is the most safe and commonly used buffer agent, lactate can be absorbed from lactate-containing PD solutions. Therefore, increases in serum lactate levels are theoretically possible if lactate clearance and metabolism are abnormal in patients treated with lactate-containing PD solutions.
The patient in this study received Hemo B Dex 0.15%-1 (JW Pharmaceutical Corporation) and B-bag powder (Fresenius Medical care Korea) as an HD solution. B-bag powder consists of 32 mEq/L sodium bicarbonate. Serum lactate level was decreased after changing the dialysis solution to one that did not contain lactate. Furthermore, serum lactate level was increased after continuing the use of a lactate-containing PD solution, but the patient's medical condition and medications did not changed. Therefore, we suspect that the lactate- containing PD solution was the main cause of lactic acidosis in this patient. This case can't be classified as definite type A or type B lactic acidosis although patient has causes of type B lactic acidosis such as diabetes and renal failure.
In normal physiological conditions, approximately 1400–1500 mmol of lactate are produced daily, primarily by the skeletal muscle, skin, and brain. Lactate clearance and metabolism occur mainly in the liver (60%) and the kidneys (30%).1 The liver was the main pathway of lactate clearance in this patient because of her ESRD. However, most PD patients do not show signs of lactic acidosis. Therefore, it is not certain why this patient had lactic acidosis caused only by the use of lactate- containing PD solution (Table 2).
The second important point to consider for lactic acidosis in this case is pyruvate metabolism. The oxidation of lactate into pyruvate by lactate dehydrogenase (LDH) is the main process for metabolic lactate clearance. The lactate-to-pyruvate ratio ranges from 4:1 to 10:1 in the normal, healthy state but ranges from 20:1 to 40:1 in patients with septic shock. The patient in this case had a pyruvate level three times higher than normal, and her lactate- to-pyruvate ratio was nearly normal range. High serum pyruvate levels may be related to increased glycolytic pathway activity due to continuous absorption of glucose in PD solution, thereby increasing pyruvate production. On the other hand, decreased activity of pyruvate dehydrogenase can induce high serum pyruvate levels in this patient. Suspected low activity of pyruvate dehydrogenase may not be related with thiamine deficiency because of normal thiamine levels, which acts as a coenzyme of pyruvate dehydrogenase. High pyruvate levels may induce increases in LDH levels, and result in increased lactate levels. Under high serum pyruvate level, it is difficult to remove overloading lactate supplied by lactate containing PD solutions by oxidation of lactate into pyruvate. Generally lactate and glucose-containing PD solutions do not induce lactic acidosis. However, in the present case, stop using lactate-containing PD solutions definitely resolved lactic acidosis and re-starting lactate-containing PD solutions definitely increased lactate levels. Therefore, we suspect that this patient has enzyme abnormalities not overcoming lactate loading in lactate or pyruvate metabolism. To our knowledge, this study is the first report of lactate-containing PD solution inducing lactic acidosis.
The use of sodium bicarbonate as a corrector for acidosis remains controversial7 because sodium bicarbonate may increase lactate production. Acidosis itself may inhibit lactic acid production by reducing activity of phosphofructokinase but sodium bicarbonate can activate of phosphofructokinase. A previous study has shown that treatment with sodium bicarbonate may negatively affect survival.8 Although a bicarbonate-containing buffer was used in this patient during HD, it did not have a significant effect on lactate levels because of the short exposure time. Further studies are needed to determine the effect of sodium bicarbonate buffers on lactic acidosis during HD.
Here, we presented a case of lactate-containing PD solution-induced lactic acidosis that was corrected by changing the treatment modality to HD. In conclusion, physicians should consider lactate- containing PD solutions as a possible cause of lactic acidosis if there is no related cause of lactic acidosis in patients treated with PD.
Figures and Tables
Fig. 2
Lactate levels according to dialysis modality.
After starting hemodialysis (2013.06.11), the lactate level was considerably reduced. After restarting peritoneal dialysis (2015.06.18), the lactate level was increased again. Therefore, we restarted hemodialysis (2015.06.23) and the lactate level was decreased again.

Table 1
Changes of Patients'acid base status

The level of bicarbonate was gradually decreased but anion gap and lactate level was gradually increased showing worsening of metabolic acidosis due to lactate. PaCO2 was severely decreased for compensation of metabolic acidosis due to increased lactate and associated respiratory alkalosis at hospital day15.
References
1. Adeva-Andany M, López Ojén M, Funcasta-Calderón R, Ameneiros-Rodriguez E, Donapetry-Garcia C, Vila-Altesor M, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014; 17:76–100.

3. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013; 88:1127–1140.

4. Keller G, Cour M, Hernu R, Illinger J, Robert D, Argaud L. Management of metformin-associated lactic acidosis by continuous renal replacement therapy. PLoS One. 2011; 6:e23200.

5. Vaziri ND, Ness R, Wellikson L, Barton C, Greep N. Bicarbonate-buffered peritoneal dialysis. An effective adjunct in the treatment of lactic acidosis. Am J Med. 1979; 67:392–396.
6. Feriani M. Buffers: bicarbonate, lactate and pyruvate. Kidney Int Suppl. 1996; 56:S75–S80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download