INTRODUCTION
Continuous ambulatory peritoneal dialysis (CAPD) is a common treatment for end-stage renal disease; currently, more than 7500 patients are maintained on peritoneal dialysis (PD) in Korea.1 Peritonitis is a frequent complication of CAPD and the most common reason for switching to hemodialysis.2 Although most PD peritonitis episodes are attributed to a single organism, most commonly coagulase-negative staphylococci,2 multiple organisms are identified in nearly 10% of peritonitis cases.3 Lactococcus lactis (L. lactis) is a gram-positive bacterium that is generally considered nonpathogenic and that is a rare cause of opportunistic infections in immunocompromised patients.4 In patients on CAPD, there have been only4 reported cases of Lactococcus-associated peritonitis.5,6,7,8 In Korea, a case of liver abscess due to L. lactis was reported in 2010;9 to our knowledge, Lactococcus-associated PD peritonitis has not been reported previously in Korea. We therefore report a case of Lactococcus-associated polymicrobial CAPD peritonitis in Korea.
CASE REPORT
A 71-year-old man who had received PD for 1 year was admitted to the emergency room for turbid peritoneal effluent with diffuse abdominal pain that had begun that day. The patient had undergone automated PD, which consisted of 4 overnight exchanges of 2 L of dialysate. He had been diagnosed with diabetes mellitus and hypertension 2 years earlier. Diabetic nephropathy with overt proteinuria was present at the time of the diagnosis, and both diabetes and hypertension had contributed to the progression to renal failure. The patient had experienced peritonitis due to Raoultella planticola 1 year previously, and the peritonitis was improved by antimicrobial treatment. On admission, his blood pressure was 140/90 mmHg, his heart rate was 84 beats/min, his respiratory rate was 20/min, and his body temperature was 36.7℃.
Examination revealed diffuse abdominal tenderness with normal bowel sounds, and neither the catheter exit site nor the tunnel sites were obviously infected. Laboratory findings were consistent with PD-associated peritonitis, including a white blood cell (WBC) count in the peritoneal effluent of 3750 cells/mm3, consisting predominantly of neutrophils (90%). The blood WBC count was 17400/mm3, with 90.4% neutrophils. C-reactive protein levels were 13.12 mg/dL (normal levels <5.0 mg/dL). Blood and peritoneal fluid cultures were performed by using blood culture bottles (BATEC). The patient was administered 1 g each cefazolin and ceftazidime into the intra-peritoneum as a loading dose, followed by a continuous dose of 0.25 g per 6 hours of cefazolin and ceftazidime into the intra-peritoneum. Gram staining of initial peritoneal fluid showed gram-positive cocci chains and gram-negative bacilli indicative of mixed infections. On the second day of hospitalization, the WBC count of the peritoneal effluent had increased to 11000 cells/mm3, with neutrophils remaining predominant (76%). Owing to the poor response to the initial antibiotics, vancomycin replaced cefazolin for broad coverage of gram-positive organisms. On day 4, the WBC count was 1150 cells/mm3 (68% neutrophils). Despite this decreased WBC count in the peritoneal fluid, it was still above 1000 cells/mm3. The patient still complained of diffuse abdominal pain and had a mild fever (37.8℃); we therefore removed the intra-peritoneal catheter. After catheter removal on day 4, we administered intravenous antibiotics, and the patient was switched from PD to hemodialysis. From day 5 onward, the patient's abdominal pain improved and his fever subsided.
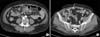
On day 4, an enhanced abdominal computed tomography scan was performed to evaluate intra-abdominal pathology as a possible route of polymicrobial peritonitis. Imaging showed mild omental infiltration and peritoneal thickening in both para-colic gutters, consistent with peritonitis; however, no other abnormal findings were detected (Fig. 1). On day 5, culture of the peritoneal dialysate revealed L. lactis, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacter cloacae. L. lactis was susceptible to vancomycin, linezolid, levofloxacin, and cefotaxime and was intermediate to penicillin. A. baumannii was susceptible to ampicillin/sulbactam, piperacillin/tazobactam, cefotaxime, and ceftazidime and resistant to cefazolin, ampicillin, and cefoxitin. P. aeruginosa was susceptible to piperacillin/tazobactam, ceftazidime, cefepime, and imipenem and resistant to ampicillin/sulbactam, cefazolin, and cefotaxime. Finally, E. cloaca was susceptible to piperacillin/tazobactam, cefotaxime, ceftazidime, cefepime, and imipenem and resistant to cefazolin, cefoxitin, and amoxicillin/clavulanate. Intravenous administration of vancomycin and ceftazidime was continued for 14 days with continued improvement in the patient's clinical symptoms. At discharge, the patient was prescribed 7 days of oral ciprofloxacin therapy. After discharge, the patient received hemodialysis treatment 3 times per week.
DISCUSSION
L. lactis is a catalase-negative gram-positive bacterium that is used in the dairy industry as a starter in cheese fermentation.4 Because it rarely causes infectious disease in humans, there are only a few reports of Lactococcus-associated infection in immunocompromised hosts, such as patients with native valve endocarditis, osteomyelitis, spondylitis, and liver abscess.10 In addition, to date, only 4 cases of Lactococcus-associated PD peritonitis have been reported: L. cremoris-associated PD peritonitis in Belgium, L. lactis in Turkey, L. cremoris in Canada, and L. garvieae in Taiwan.5,6,7,8 In Korea, consumption of dairy products has increased recently reflecting rising incomes and Westernized diets; therefore, there may be an increased risk of Lactococcus-associated infectious disease, especially in immune-deficient patients. In our case, the patient frequently ate and handled butter without washing his hands before dressing his exit site and handling the peritoneal dialysate. Therefore, contamination during exchange was a possible route of infection. Owing to the presence of both gram-negative and gram-positive organisms in this case, we also considered intra-abdominal disease and transluminal contamination. Although the abdominal computed tomography scan showed no obvious causes of the intra-abdominal lesions except peritonitis, invisible lesions such as micro-perforations are a possible source of transmission, because Lactococcus, along with other Enterobacteriaceae, are part of the normal gastrointestinal tract flora. Obvious abdominal lesions have been reported to be present in less than 9% of polymicrobial peritonitis cases, even with suspected gastrointestinal sources of infection.3
There is currently no standard treatment protocol for Lactococcus infections; they are usually successfully managed by using protocols recommended for streptococcal infections. In previous reports of Lactococcus-associated PD peritonitis, good responses to antibiotics such as cefazolin and vancomycin were observed.5,6,7,8 However, as in our experience, recent epidemiological studies have found polymicrobial peritonitis to have higher incidences of antibiotic resistance, catheter removal, and hemodialysis transfer compared with single-organism infections. In addition, compared with gram-positive infections, mixed gram-negative or fungal-associated peritonitis has a worse prognosis and generally high rates of early catheter removal.3 In our case, switching to vancomycin for treatment of gram-positive bacteria resulted in an improved treatment response, which suggests an important role of Lactococcus in disease progression. However, broad coverage using vancomycin and ceftazidime was not enough to improve the disease. Therefore, we removed the PD catheter and switched the therapy to hemodialysis. Although L. lactis was an important pathogen and was susceptible to vancomycin, polymicrobial infection resulted in refractory peritonitis.
In conclusion, to our knowledge, this is the first report of polymicrobial peritonitis associated with L. lactis in Korea. Although it is rare, we need to consider L. lactis as a potential pathogen of PD peritonitis, especially considering the growing exposure in Korea to Western diets that include dairy products.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download