1. Henley SJ, Dowling NF, Ahmad FB, Ellington TD, Wu M, Richardson LC. COVID-19 and other underlying causes of cancer deaths - United States, January 2018-July 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(50):1583–1588. PMID:
36520660.

2. Park JM, Koo HY, Lee JR, Lee H, Lee JY. COVID-19 mortality and severity in cancer patients and cancer survivors. J Korean Med Sci. 2024; 39(2):e6. PMID:
38225782.

3. Trapani D, Curigliano G. COVID-19 vaccines in patients with cancer. Lancet Oncol. 2021; 22(6):738–739. PMID:
34087120.

4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384(22):2092–2101. PMID:
33835769.

5. Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker V, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med. 2022; 19(2):e1003926. PMID:
35192597.

6. Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021; 375:e068665. PMID:
34916207.

7. Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021; 398(10306):1147–1156. PMID:
34370972.

8. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384(22):2124–2130. PMID:
33835768.

9. Wong HL, Hu M, Zhou CK, Lloyd PC, Amend KL, Beachler DC, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022; 399(10342):2191–2199. PMID:
35691322.

10. Jiang J, Chan L, Kauffman J, Narula J, Charney AW, Oh W, et al. Impact of vaccination on major adverse cardiovascular events in patients with COVID-19 infection. J Am Coll Cardiol. 2023; 81(9):928–930. PMID:
36813689.

11. Fernandes CJ, Morinaga LT, Alves JL Jr, Castro MA, Calderaro D, Jardim CV, et al. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev. 2019; 28(151):28.

12. Fendler A, de Vries EG, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022; 19(6):385–401. PMID:
35277694.

14. Nham E, Song JY, Noh JY, Cheong HJ, Kim WJ. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci. 2022; 37(47):e351. PMID:
36472087.

15. Waldhorn I, Holland R, Goshen-Lago T, Shirman Y, Szwarcwort-Cohen M, Reiner-Benaim A, et al. Six-month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 2021; 11(10):2430–2435. PMID:
34475136.

16. Javadinia SA, Alizadeh K, Mojadadi MS, Nikbakht F, Dashti F, Joudi M, et al. COVID-19 vaccination in patients with malignancy; a systematic review and meta-analysis of the efficacy and safety. Front Endocrinol (Lausanne). 2022; 13:860238. PMID:
35586627.

17. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020; 395(10241):1907–1918. PMID:
32473681.
18. Hwang JK, Zhang T, Wang AZ, Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021; 14(1):38. PMID:
33640005.

19. Pinato DJ, Tabernero J, Bower M, Scotti L, Patel M, Colomba E, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021; 22(12):1669–1680. PMID:
34741822.
20. Lozahic C, Maddock H, Sandhu H. Anti-cancer therapy leads to increased cardiovascular susceptibility to COVID-19. Front Cardiovasc Med. 2021; 8:634291. PMID:
33969006.

21. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21(3):335–337. PMID:
32066541.

22. Burn E, Li X, Delmestri A, Jones N, Duarte-Salles T, Reyes C, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in the United Kingdom. Nat Commun. 2022; 13(1):7167. PMID:
36418291.

23. Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021; 181(12):1668–1670. PMID:
34605853.

24. Le Vu S, Bertrand M, Jabagi MJ, Botton J, Drouin J, Baricault B, et al. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat Commun. 2022; 13(1):3633. PMID:
35752614.

25. Bae DH, Kim M, Lee DI, Lee JH, Kim S, Lee SY, et al. Simultaneous occurrence of immune-mediated thrombocytopenia and myocarditis after mRNA-1273 COVID-19 vaccination: a case report. J Korean Med Sci. 2022; 37(21):e169. PMID:
35638196.

26. Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol. 1996; 143(11):1165–1173. PMID:
8633607.

27. Weldeselassie YG, Whitaker HJ, Farrington CP. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011; 139(12):1805–1817. PMID:
21849099.

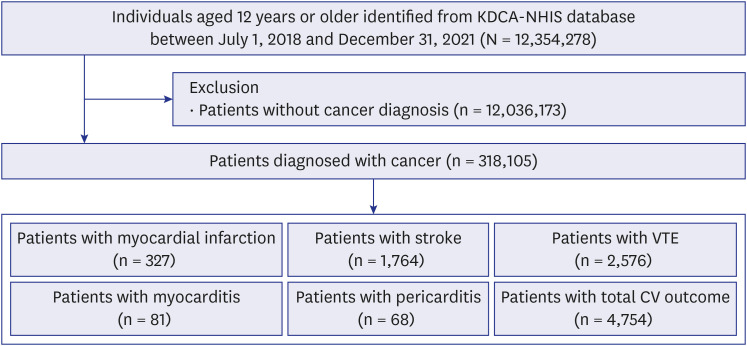
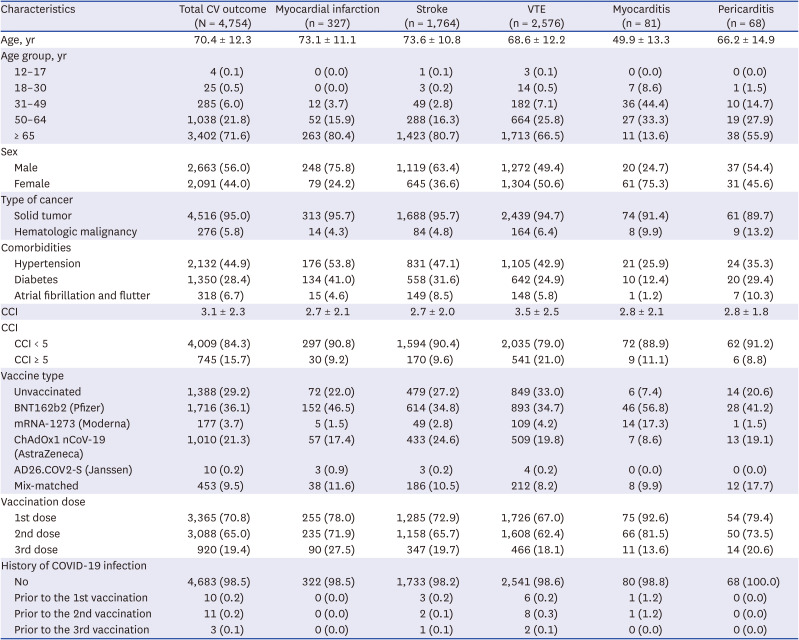
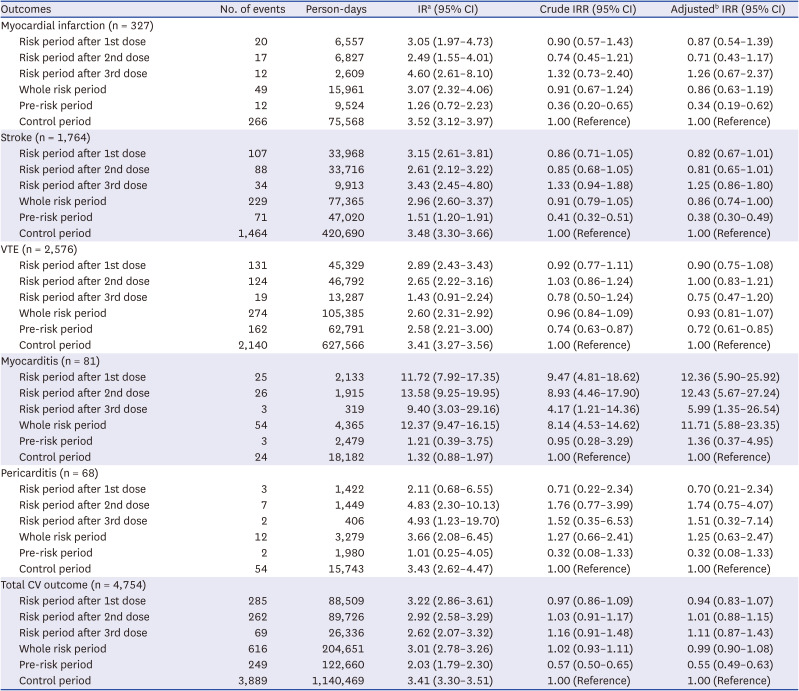




 PDF
PDF Citation
Citation Print
Print



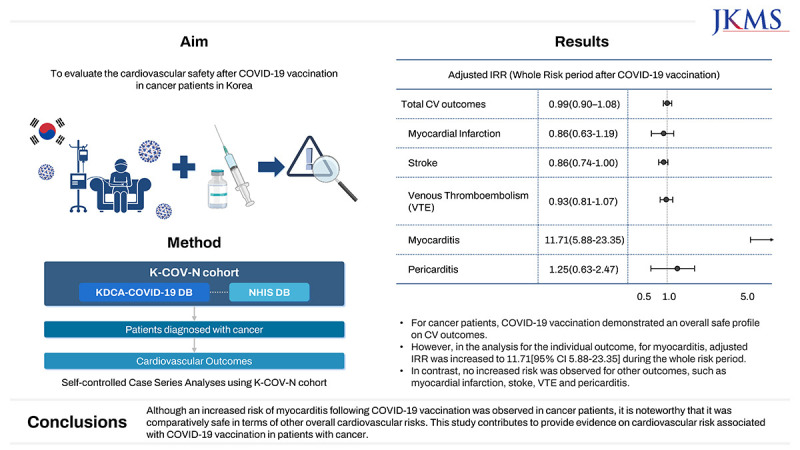
 XML Download
XML Download