1. Daghlas I, Dashti HS, Lane J, et al. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019; 74:1304–1314.

2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010; 33:585–592.

3. Barnes A, Mountifield R, Baker J, et al. A systematic review and meta-analysis of the prevalence of poor sleep in inflammatory bowel disease. Sleep Adv. 2022; 3:zpac025.

4. Ballesio A, Zagaria A, Baccini F, Micheli F, Di Nardo G, Lombardo C. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med Rev. 2021; 60:101518.

5. Hao G, Zhu B, Li Y, Wang P, Li L, Hou L. Sleep quality and disease activity in patients with inflammatory bowel disease: a systematic review and meta-analysis. Sleep Med. 2020; 75:301–308.

6. Iskandar HN, Linan EE, Patel A, et al. Self-reported sleep disturbance in Crohn’s disease is not confirmed by objective sleep measures. Sci Rep. 2020; 10:1980.

7. Gîlc-Blanariu GE, Ștefnescu G, Trifan AV, et al. Sleep impairment and psychological distress among patients with inflammatory bowel disease-beyond the obvious. J Clin Med. 2020; 9:2304.

8. Scott AJ, Flowers O, Rowse G. Do specific types of sleep disturbances represent risk factors for poorer health-related quality of life in inflammatory bowel disease? A longitudinal cohort study. Br J Health Psychol. 2021; 26:90–108.

9. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses. Part I. Inflamm Bowel Dis. 2018; 24:742–751.

10. Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011; 17:1882–1889.

11. Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013; 11:965–971.

12. Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014; 12:1315–23.e2.

13. Uemura R, Fujiwara Y, Iwakura N, et al. Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. Springerplus. 2016; 5:1792.

14. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Sleep duration affects risk for ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014; 12:1879–1886.

15. Salwen-Deremer JK, Smith MT, Haskell HG, Schreyer C, Siegel CA. Poor sleep in inflammatory bowel disease is reflective of distinct sleep disorders. Dig Dis Sci. 2022; 67:3096–3107.

16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007; 30:213–218.

17. Koyanagi A, Garin N, Olaya B, et al. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. PLoS One. 2014; 9:e114742.

18. Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998; 158:1099–1107.

19. Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006; 12:S221–S229.
20. Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007; 16:85–95.

21. Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005; 39:151–159.

22. Schwartz S, McDowell Anderson W, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999; 47:313–333.
23. Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002; 251:207–216.

24. Novak M, Mucsi I, Shapiro CM, Rethelyi J, Kopp MS. Increased utilization of health services by insomniacs: an epidemiological perspective. J Psychosom Res. 2004; 56:527–536.

25. Léger D, Massuel MA, Metlaine A; SISYPHE Study Group. Professional correlates of insomnia. Sleep. 2006; 29:171–178.
26. Salwen-Deremer JK, Smith MT, Aschbrenner KA, Haskell HG, Speed BC, Siegel CA. A pilot feasibility trial of cognitive-behavioural therapy for insomnia in people with inflammatory bowel disease. BMJ Open Gastroenterol. 2021; 8:e000805.

27. Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: a systematic review with meta-analysis. Saudi J Gastroenterol. 2018; 24:141–150.

28. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011; 31:180–186.

29. Frigstad SO, Høivik ML, Jahnsen J, et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J Gastroenterol. 2018; 24:3293–3301.

30. Gingold-Belfer R, Peled N, Levy S, et al. Impaired sleep quality in Crohn’s disease depends on disease activity. Dig Dis Sci. 2014; 59:146–151.

31. Marinelli C, Savarino EV, Marsilio I, et al. Sleep disturbance in inflammatory bowel disease: prevalence and risk factors: a cross-sectional study. Sci Rep. 2020; 10:507.
32. Keskin EB, Şahbaz CD. Chronotype and sleep quality in patients with inflammatory bowel disease. Med Bull Haseki. 2020; 58:72–77.

33. Sochal M, Małecka-Panas E, Gabryelska A, et al. Determinants of sleep quality in inflammatory bowel diseases. J Clin Med. 2020; 9:2921.

34. Sofia MA, Lipowska AM, Zmeter N, Perez E, Kavitt R, Rubin DT. Poor sleep quality in Crohn’s disease is associated with disease activity and risk for hospitalization or surgery. Inflamm Bowel Dis. 2020; 26:1251–1259.

35. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34:601–608.

36. Blais FC, Gendron L, Mimeault V, Morin CM. Evaluation of insomnia: validity of 3 questionnaires. Encephale. 1997; 23:447–453.
37. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2:297–307.

38. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980; 1:514.

39. Bennebroek Evertsz’ F, Hoeks CC, Nieuwkerk PT, et al. Development of the patient Harvey Bradshaw Index and a comparison with a clinician-based Harvey Bradshaw Index assessment of Crohn’s disease activity. J Clin Gastroenterol. 2013; 47:850–856.

40. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998; 43:29–32.

41. Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013; 7:890–900.

42. Marín-Jiménez I, Nos P, Domènech E, et al. Diagnostic performance of the simple clinical colitis activity index self-administered online at home by patients with ulcerative colitis: CRONICA-UC study. Am J Gastroenterol. 2016; 111:261–268.

43. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003; 35:1381–1395.

44. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006; 166:1092–1097.

45. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606–613.
46. Paulides E, Kim C, Frampton C, et al. Validation of the inflammatory bowel disease disability index for self-report and development of an item-reduced version. J Gastroenterol Hepatol. 2019; 34:92–102.

47. Gower-Rousseau C, Sarter H, Savoye G, et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. 2017; 66:588–596.

48. Mustafa M, Bawazir Y, Merdad L, et al. Frequency of sleep disorders in patients with rheumatoid arthritis. Open Access Rheumatol. 2019; 11:163–171.
49. Reynolds AC, Appleton SL, Gill TK, Adams RJ. Chronic insomnia disorder in Australia. Sleep Health Foundation;North Strathfield: 2019.
50. Sivertsen B, Pallesen S, Friborg O, et al. Sleep patterns and insomnia in a large population-based study of middle-aged and older adults: the Tromsø study 2015-2016. J Sleep Res. 2021; 30:e13095.

51. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002; 6:97–111.

52. Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013; 154:1613–1621.

53. Cremonini F, Camilleri M, Zinsmeister AR, Herrick LM, Beebe T, Talley NJ. Sleep disturbances are linked to both upper and lower gastrointestinal symptoms in the general population. Neurogastroenterol Motil. 2009; 21:128–135.

54. Ellis JG, Perlis ML, Bastien CH, Gardani M, Espie CA. The natural history of insomnia: acute insomnia and first-onset depression. Sleep. 2014; 37:97–106.

55. Cox RC, Olatunji BO. Sleep in the anxiety-related disorders: a meta-analysis of subjective and objective research. Sleep Med Rev. 2020; 51:101282.

56. Sylvia LG, Dupuy JM, Ostacher MJ, et al. Sleep disturbance in euthymic bipolar patients. J Psychopharmacol. 2012; 26:1108–1112.

57. van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, van Straten A. Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies. Sleep Med Rev. 2019; 48:101208.

58. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015; 163:191–204.
59. Sadler P, McLaren S, Klein B, Harvey J, Jenkins M. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018; 41:zsy104.

60. Manber R, Buysse DJ, Edinger J, et al. Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized controlled trial. J Clin Psychiatry. 2016; 77:e1316–e1323.
61. McCurry SM, Zhu W, Von Korff M, et al. Effect of telephone cognitive behavioral therapy for insomnia in older adults with osteoarthritis pain: a randomized clinical trial. JAMA Intern Med. 2021; 181:530–538.

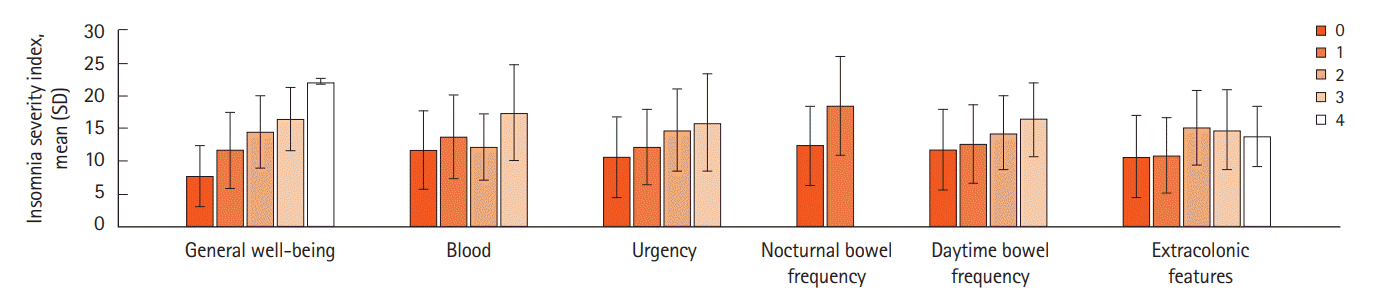
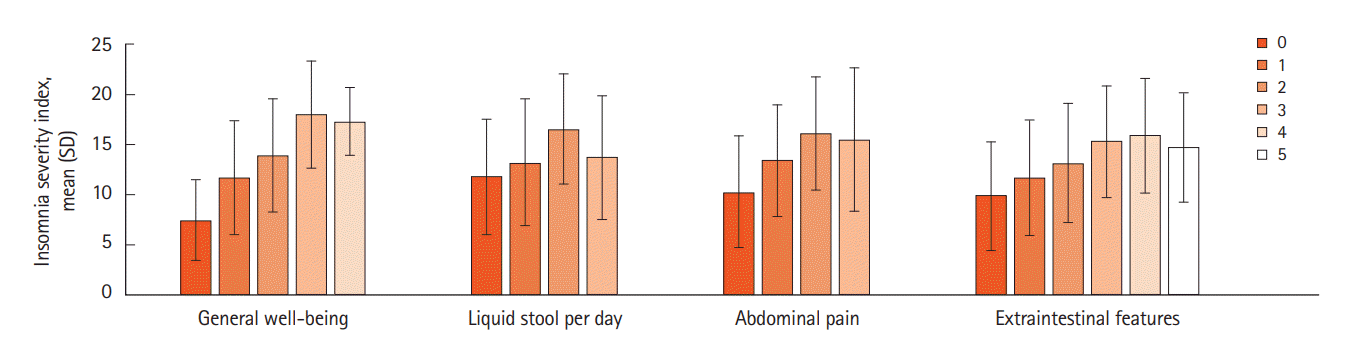




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download