INTRODUCTION
Although there is controversy regarding the use of magnesium sulfate (MgSO
4) as a tocolytic agent, MgSO
4 has been widely used to treat preterm labor, preeclampsia, and eclampsia.
1 Recently, the fetal neuroprotective effect of antenatal MgSO
4 has emerged, and several meta-analyses have emphasized this effect. In a meta-analysis of five randomized controlled trials (RCTs), antenatal MgSO
4 use was associated with a decreased risk of cerebral palsy and substantial gross motor dysfunction, defined as an inability to walk alone, although there was no significant effect on infant mortality.
2 In another meta-analysis by Costantine et al.
3 with six RCTs (five studies), antenatal MgSO
4 decreased the risk of moderate-to-severe cerebral palsy without decreasing the incidence of death or cerebral palsy. Furthermore, in another meta-analysis by Conde-Agudelo and Romero.
4 with the same six RCTs as Costantine et al.’s study,
3 they concluded that antenatal MgSO
4 was associated with a decreased risk of cerebral palsy, moderate-to-severe cerebral palsy, and substantial gross motor dysfunction, although cerebral palsy decreased in one RCT. Subsequently, in 2010, the American College of Obstetricians and Gynecologists (ACOG) encouraged the administration of antenatal MgSO
4 to reduce the risk of cerebral palsy in pregnant woman with imminent preterm birth at gestational age (GA) less than 32 weeks based on the results of several multicenter RCTs and the above-mentioned meta-analysis.
5 In 2017, the ACOG reached a consensus that antenatal MgSO
4 is recommended as early as GA 24–25 weeks, considered for GA 23 weeks, and not recommended for GA 20–22 weeks.
6 In 2019, the Society of Obstetricians and Gynaecologists of Canada released a practice guideline that antenatal MgSO
4 should be considered in pregnant women with imminent preterm birth at less than 34 weeks of GA without fear of overuse, for fetal neuroprotection.
7
Suspicions about the long-term neuroprotective effect of antenatal MgSO
4 have been raised after several years of use for neuroprotection. There were two school-age follow-up studies out of the above-mentioned studies of antenatal MgSO
4 treatment, which could not find a benefit of antenatal MgSO
4 treatment at school age. The enrolled preterm infants in a large multicenter RCT by Crowther et al.
8 were followed up till school age of 6–11 years, and antenatal MgSO
4 was not found to be associated with improved cognitive, behavioral, growth, or functional outcomes at school age.
9 Furthermore, in another follow-up study of a French RCT,
10 there were no significant differences in motor impairment, cognitive impairment, behavioral disorder, or mortality between the MgSO
4 and placebo groups at the school age of 7–14 years.
11
Together with the discrepancy regarding long-term neuroprotective effects of antenatal MgSO
4, there has been concerns regarding fetal and neonatal safety of antenatal MgSO
4 with aggressive treatment by the recommendations of ACOG and other committees. Safety issues included decreased mesenteric blood flow and intestinal motility resulting in meconium-related ileus, necrotizing enterocolitis (NEC) or spontaneous intestinal perforation.
12 Researchers suggested that suspicion of gastrointestinal complications in particular is required, especially in preterm infants with a GA < 26 weeks exposed to antenatal MgSO
4 until sturdy evidence of its association could be established.
12 The protocol of administering antenatal MgSO
4 for fetal neuroprotection was adopted in our institute in January 2014. However, although we have shown that there was no significant association between antenatal MgSO
4 use and subsequent NEC development in preterm infants with GA of 24 to 31 weeks
13 there were gastrointestinal complications such as meconium-related ileus in extremely preterm infants with GA of ≤ 25 weeks, that lead to withdrawal of the protocol in March 2016. In our previous study, antenatal MgSO
4 increased the risk of meconium-related ileus requiring surgical treatment in preterm infants with a GA of ≤ 25 weeks.
14 Furthermore, antenatal MgSO
4 did not have neuroprotective effects assessed at corrected age (CA) of 18–24 months in infants with a GA of ≤ 25 weeks.
14
Despite these concerns and debates, antenatal MgSO4 is already used worldwide, based on the recommendations of the ACOG and other committees for fetal neuroprotection. Therefore, this study aimed to investigate whether antenatal MgSO4 for fetal neuroprotection was clearly associated with improved long-term neurodevelopmental and growth outcomes at CA of 18–24 months and 3 years of age without significant side effects in preterm infants with a GA of 24–31 weeks who are at great risk for long-term neurodevelopmental and growth impairment.
METHODS
Medical records of very low birth weight infants weighing less than 1,500 g with GA 24–31 weeks who were admitted to the Samsung Medical Center neonatal intensive care unit (NICU) between January 2012 and March 2018 and were followed up at CA of 18–24 months (follow-up 1) or at 3 years of age (follow-up 2) were reviewed.
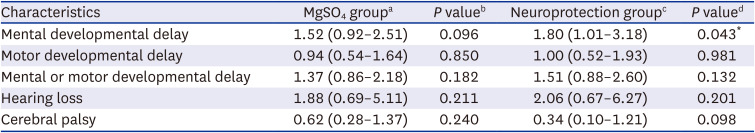
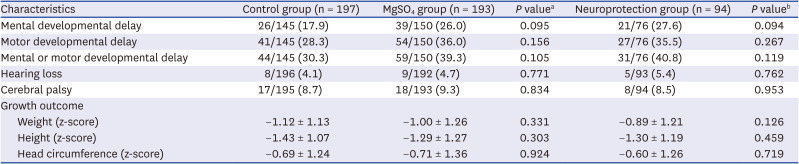
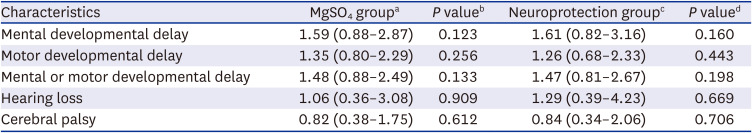
Patient characteristics and long-term neurodevelopmental and growth outcomes were compared between two groups. Infants who were exposed to antenatal MgSO4 for any purpose (MgSO4 group) were compared with infants who were not exposed to antenatal MgSO4 (control group). Additionally, infants who were exposed to antenatal MgSO4 for fetal neuroprotection (neuroprotection group) were compared with the control group.
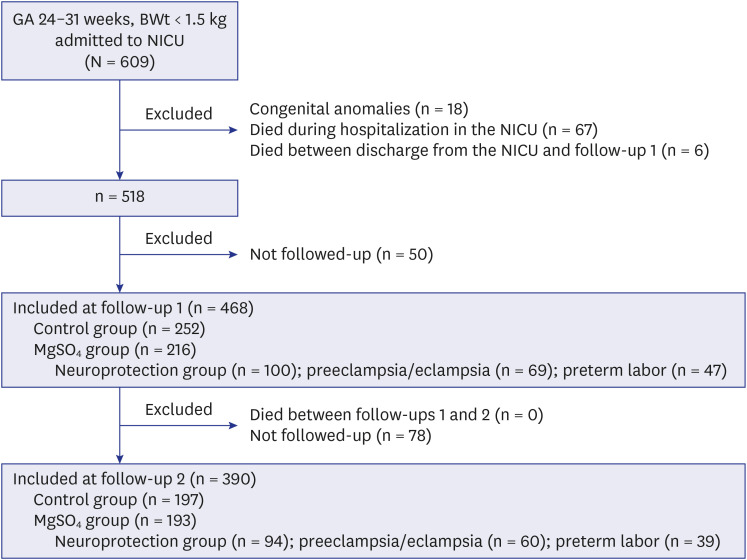
Among the 609 preterm infants, 18 with congenital anomalies, 67 who died during hospitalization in the NICU, and 6 who died between discharge from the NICU and follow-up 1 were excluded (
Fig. 1). Among the surviving 518 eligible infants, 50 were not followed up at a CA of 18–24 months (follow-up 1); hence, a total of 468 infants were included at follow-up 1. Among these 468 infants, 252 were not exposed to MgSO
4 (control group) and 216 were exposed to MgSO
4 (MgSO
4 group) (100 infants for fetal neuroprotection [neuroprotection group], 69 infants to treat maternal preeclampsia or eclampsia, and 47 infants to treat maternal preterm labor). At 3 years of age (follow-up 2), among 468 infants who were included at follow-up 1, 78 were not followed up. None of the infants died during follow-ups 1 and 2. Finally, 390 infants were included at follow-up 2. Among these 390 infants, 197 were not exposed to MgSO
4 (control group) and 193 were exposed to MgSO
4 (MgSO
4 group) (94 infants for fetal neuroprotection [neuroprotection group], 60 infants to treat maternal preeclampsia or eclampsia, and 39 infants to treat maternal preterm labor).
Fig. 1
Patient flowchart. Among 609 preterm infants weighing < 1,500 g with GA 24–31 weeks who were admitted from January 2012 to March 2018, 18 with congenital anomalies, 67 who died during hospitalization, and 6 who died between discharge from the neonatal intensive care unit and follow-up 1 were excluded. Among the surviving 518 eligible infants, 50 were not followed-up; hence, a total of 468 infants were included at corrected age of 18–24 months (follow-up 1). Among these 468 infants, 252 were not exposed to MgSO4 (control group) and 216 were exposed to MgSO4 (MgSO4 group) (100 infants for fetal neuroprotection [neuroprotection group], 69 infants to treat maternal preeclampsia or eclampsia, 47 infants to treat maternal preterm labor). At 3 years of age (follow-up 2), among 468 infants who were included at follow-up 1, 78 were not followed-up. None of the infants died between follow-ups 1 and 2. Finally, a total of 390 infants were included at follow-up 2. Among these 390 infants, 197 were not exposed to MgSO4 (control group) and 193 were exposed to MgSO4 (MgSO4 group) (94 infants for fetal neuroprotection [neuroprotection group], 60 infants to treat maternal preeclampsia or eclampsia, and 39 infants to treat maternal preterm labor).
GA = gestational age, BWt = birth weight, NICU = neonatal intensive care unit, MgSO4 = magnesium sulfate.

Antenatal MgSO4 for fetal neuroprotection was given to women with imminent preterm birth as a 4-g bolus followed by an infusion of 1 g of MgSO4 per hour for 24 hours. Maintenance dose was discontinued if delivery did not occur within 24 hours. MgSO4 for the treatment of preeclampsia and eclampsia was given as the same loading dose of 4-g bolus followed by an infusion of 1 g of MgSO4 per hour and discontinued at 24 hours after delivery. All women who received MgSO4 for tocolysis were transferred from another hospital because MgSO4 was not used as a tocolytic agent in our institute.
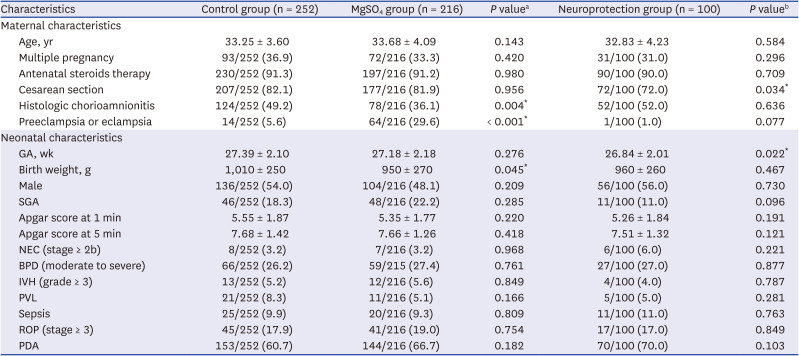
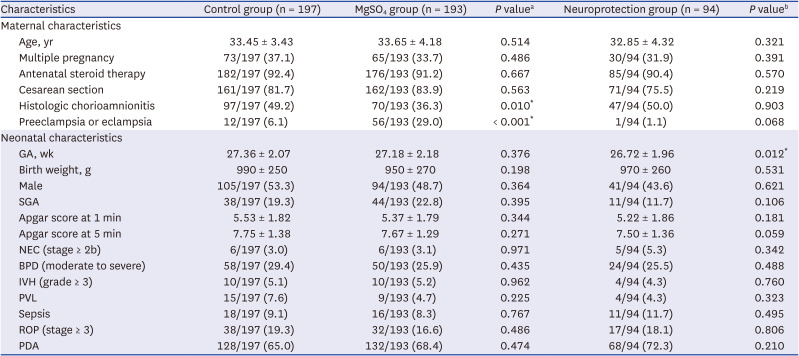
Maternal characteristics included maternal age, antenatal steroid therapy, antenatal MgSO
4 treatment, intent of MgSO
4 treatment, mode of delivery, and histologic chorioamnionitis. Neonatal characteristics included GA, birth weight, sex, small for GA, Apgar scores at 1 and 5 min, NEC (stage ≥ 2b),
15 bronchopulmonary dysplasia (moderate-to-severe) according to the National Institute of Child Health and Human Development 2001 definition,
16 intraventricular hemorrhage (grade ≥ 3),
17 periventricular leukomalacia, culture-proven sepsis, retinopathy of prematurity (stage ≥ 3),
18 and patent ductus arteriosus.
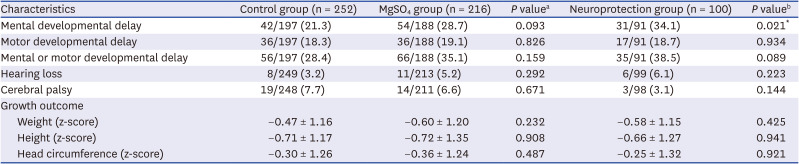
Long-term outcomes of neurodevelopment and growth were assessed at the follow-ups 1 and 2. Mental developmental delay was defined as 1) a mental developmental index < 70 on the Bayley Scales of Infant Development (BSID) II; 2) cognitive or language < 70 on the BSID III; or 3) cognition, language, or self-help score less than the cut-off value in the Korean Developmental Screening Test for Infants and Children (K-DST) in patients who were conducted K-DST without BSID.
19 It has been shown that the results of the K-DST are well correlated with those of BSID data, supporting the reliability and validity of the K-DST for assessing neurodevelopmental disorders in Korean infants.
202122 Motor developmental delay was defined as 1) a psychomotor developmental index < 70 on the BSID II; 2) motor < 70 on the BSID III; or 3) gross motor or fine motor score less than the cut-off value on the K-DST in patients who were conducted K-DST without BSID.
19 Hearing loss was defined as sensorineural hearing loss requiring a hearing aid or cochlear implantation, as diagnosed by a pediatric otolaryngologist. Cerebral palsy was defined as a non-progressive movement disorder diagnosed by a physician specializing in pediatric rehabilitation medicine with Gross Motor Functional Classification System levels ≥ 2.
23 Z-scores of weight, height, and head circumference according to the 2006 World Health Organization Child Growth Standards were compared.
24
Statistical analysis
The t-test or Mann-Whitney U test was performed for continuous variables with a normal distribution and homogeneous variance, and the χ2 test or Fisher’s exact test was performed for categorical variables, as appropriate. Odds ratios (ORs) for the MgSO4 group were adjusted for GA, birth weight, maternal histologic chorioamnionitis, and preeclampsia/eclampsia, which differed between the MgSO4 and control groups and might have affected long-term neurodevelopmental and growth outcomes. ORs for the neuroprotection group were adjusted for GA, birth weight, and cesarean section, and were different between the neuroprotection and control groups. Adjusted OR and 95% confidence interval compared to the control group using multivariable logistic regression were presented to evaluate the risks of long-term neurodevelopmental impairment, or growth restriction. All statistical analyses were performed using STATA (version 16.0; StataCorp, College Station, TX, USA). Data are presented as mean ± standard deviation for continuous variables and as numbers (percentages) for categorical variables, and P values < 0.05 were considered significant.
Ethics statement
This study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (identification code:2022-08-080) in accordance with the Declaration of Helsinki. The Institutional Review Board waived the need for informed consent for this retrospective chart review.
DISCUSSION
In the present study, antenatal MgSO4 including MgSO4 for neuroprotection was not associated with short-term morbidities in the NICU, namely, NEC, intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, or sepsis. Furthermore, antenatal MgSO4 including MgSO4 for neuroprotection was not associated with improved long-term neurodevelopmental and growth outcomes at CA of 18–24 months and 3 years of age.
Although Crowther et al.
8 recommended MgSO
4 to improve outcomes in preterm infants, the total mortality of enrolled preterm infants with GA < 30 weeks, cerebral palsy at CA of 2 years in survivors, and combined death or cerebral palsy did not significantly decrease in the MgSO
4 group compared to the placebo group by RCT at 16 tertiary hospitals in Australia and New Zealand. They reported that substantial gross motor dysfunction defined as an inability to walk alone and combined death or substantial gross motor dysfunction decreased.
8
Further, in an RCT by Marret et al.
10 (PREMAG trial) at 18 tertiary hospitals in France, there were no statistically significant differences in total mortality, severe white matter injury, and combined outcomes between the MgSO
4 and placebo groups among preterm infants with GA < 33 weeks. In a 2-year follow-up of the PREMAG trial, cerebral palsy, death, and combined death and cerebral palsy did not decrease significantly in the MgSO
4 group; however, death and gross motor dysfunction were significantly less frequent in the MgSO
4 group than in the placebo group.
25 In another multicenter trial in the US at 20 hospitals in Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, Rouse et al.
26 found no statistical differences in long-term developmental outcomes measured by the BSID and mortality between the MgSO
4 and placebo groups among preterm infants with GA 24–31 weeks. However, the authors concluded that moderate or severe cerebral palsy was less frequent in the MgSO
4 group than in the placebo group, suggesting that MgSO
4 may reduce the risk of cerebral palsy in preterm infants who are at a high risk for cerebral palsy, although there was no significant effect of MgSO
4. In contrast, exposure to antenatal MgSO
4 was associated with worse outcomes in infants; therefore, Mittendorf et al.
27 recommended abandoning routine use of antenatal MgSO
4 as a tocolytic agent. In a follow-up study of the Magpie Trial with 125 centers from 19 countries,
28 there were no differences in death, blindness, deafness, severe cerebral palsy, or developmental delay at 18 months of age between infants who were antenatally exposed to MgSO
4 and those who were not exposed.
The ACOG committee opinion in 2010 was mainly based on the aforementioned large multicenter trials by Crowther et al.,
8 Marret et al.,
1025 Rouse et al.,
26 and Mittendorf et al.
27 although the results of these RCTs were inconclusive or even negative. Moreover, systematic reviews and meta-analyses
234 which the ACOG committee opinion is based on, overlap, and include the same clinical trials. Furthermore, the ACOG committee recognized that none of the included individual studies found a benefit in terms of the primary outcome. Nonetheless, the ACOG committee’s opinion of antenatal MgSO
4 for neuroprotection was released, and antenatal MgSO
4 is already being used worldwide according to the recommendations of the ACOG and other committees.
57
Since antenatal MgSO
4 was aggressively administered according to the recommendations of the ACOG and other committees, there have been concerns about the long-term neuroprotective effect of antenatal MgSO
4 and fetal and neonatal safety. In the present study, antenatal MgSO
4 including MgSO
4 for neuroprotection did not reduce the risk of neurodevelopmental impairment, hearing loss, or cerebral palsy at CA of 18–24 months and 3 years of age. Rather, more infants had delayed mental development at CA of 18–24 months who were exposed to antenatal MgSO
4 for neuroprotection than those who were not exposed to antenatal MgSO
4. In a recent meta-analysis of 11 studies (six RCTs and five cohort studies), antenatal MgSO
4 did not reduce the risk of cerebral palsy, death, long-term gross motor dysfunction, blindness, deafness, or developmental delay, which reduced the risk of moderate to severe cerebral palsy.
29 In a cohort study of preterm infants born with GA ≥ 30 weeks exposed to antenatal MgSO
4, it did not seem to promote white matter microstructure development, which affects motor or cognitive functions.
30 Furthermore, NEC was more frequent without statistical significance in infants with the primary intent of MgSO
4 being fetal neuroprotection in the present study. In a recent meta-analysis of five studies, NEC was more frequent without statistical significance, and developmental delay and gross motor dysfunction did not decrease in the MgSO
4 group, which is consistent with our results.
31 The authors found that combined death or cerebral palsy decreased in infants with the primary intent of MgSO
4 being fetal neuroprotection, and the risk of cerebral palsy decreased in infants with GA < 28 weeks.
31
In contrast, our previous study with antenatal MgSO
4 for fetal neuroprotection was not associated with NEC or mortality in preterm infants with GA 24–31 weeks.
13 In another meta-analysis of six trials, antenatal MgSO
4 administration decreased the risk of cerebral palsy without increasing the risk of mortality.
32 In a 9-year prospective study, the prevalence of cerebral palsy at 2 years of age in preterm infants with GA < 33 weeks decreased after (2010–2015) the adoption of MgSO
4 for fetal neuroprotection compared to before (2007–2009).
33 However, in that study by Chollat et al.,
33 the prevalence of cerebral palsy was not analyzed by direct comparison of infants exposed to antenatal MgSO
4 or not. In addition, the decreased prevalence of cerebral palsy may be related to improvements in NICU care. Therefore, caution must be exercised when interpreting the relationship between antenatal MgSO
4 use and cerebral palsy.
Finally, most of the recent studies regarding the neuroprotective effect of antenatal MgSO4 in preterm infants are inconclusive; therefore, these studies are unpersuasive. Despite this, the neuroprotective effect of antenatal MgSO4 administration in preterm infants has been highlighted. Further rigorous research with a multicenter large cohort is needed to determine the safety and long-term neuroprotective effects of antenatal MgSO4.
The present study has certain limitations. Firstly, the risk of mental developmental delay was higher in the neuroprotection group compared to the control group. Furthermore, the risk of developmental delay was higher in both the MgSO
4 group and the neuroprotection group among infants with a GA age of 28 weeks or more (
Supplementary Tables 5 and
6). Nevertheless, it is not fully explained due to the limitations of a retrospective single-center study and the lack of complete follow-up for all infants. Second, we utilized multiple tools for developmental testing, namely BSID II and BSID III as a confirmatory test, and K-DST as a screening test for developmental delay. It is important to note that BSID II and III differ in their assessment methods and scoring systems, which introduces a potential source of error in our study. Despite our efforts to develop composite data in order to maximize the utilization of available data and minimize variability and selection bias, it is important to acknowledge that bias may still be present due to the inherent inter-test variability. Third, we applied the same cut-off value for both BSID II and BSID III as other researchers.
14193435 However, there is still ongoing debate as different studies have used varying cut-off values.
363738 Therefore, this remains a topic of controversy.
However, the strength of this study is the validity of maternal and neonatal data with good data management by blinding chart reviewers. Furthermore, the treatment policy and assessment of neurodevelopment by the attending physician were uniform and consistent without inter-center variability, with the benefit of a single-center study. In addition, follow-up rates were higher with 90.3% (at follow-up 1) and 75.3% (at follow-up 2) compared with multicenter nationwide studies with those of 74.6% (at follow-up 1) and 57.7% (at follow-up 2) by the Korean Neonatal Network, including > 70 NICUs.
3940 In addition, the rate of neurodevelopmental assessments was high in this study. A total of 82.3% (385/468) and 75.6% (295/390) of the enrolled preterm infants underwent the BSID II, BSID III, or K-DST at follow-ups 1 and 2, respectively.
In conclusion, this study found no beneficial effects of antenatal MgSO4, including MgSO4 for neuroprotection, on short-term outcomes in preterm infants weighing < 1,500 g with GA 24–31 weeks. Furthermore, it showed no beneficial effects on long-term neurodevelopmental and growth outcomes at CA of 18–24 months and 3 years of age, contrary to previous findings.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download