INTRODUCTION
Coronavirus disease 2019 (COVID-19) has been associated with a wide disease spectrum, ranging from asymptomatic to severe pneumonia.
12 Although prompt and appropriate treatment has been shown to affect the prognosis of patients with novel infectious diseases,
3 ineffective medications may negatively affect prognosis due to their potential adverse events. Evidence-based information should therefore be sufficiently distributed at an appropriate time, including when information on novel infectious diseases is limited. Front-line medical staff require appropriate and properly distributed clinical guidelines to care for patients.
Transnational organizations such as the World Health Organization (WHO), individual countries, and related academic societies have published clinical guidelines for COVID-19 since the early period of the pandemic in 2020. The first interim guidance published by the WHO for COVID-19 treatment in January 2020 did not include a specific recommendation for drug use,
4 although later guidelines in November 2020 conditionally recommended remdesivir for the treatment of COVID-19 pneumonia.
5 The U.S. Centers for Disease Control and Prevention (CDC) and the U.S. National Institutes of Health (NIH) have issued clinical guidelines starting in April 2020,
6 which have since been revised irregularly and repeatedly to facilitate access to the latest medical recommendations. The National Institute for Health and Care Excellence in the United Kingdom published rapid guidelines for COVID-19 beginning in April 2021.
7 In Korea, the basic initial medical guidelines were published in March 2020, with later compiled guidelines published in November 2020, leaving a 7 month gap in treatment guidelines.
89
Research into the development and repositioning of drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) found that some antiviral candidates were ineffective.
10 For example, the U.S. Food and Drug Administration (FDA) approved hydroxychloroquine (HCQ) for emergency use on March 28, 2020, but this approval was revoked on June 15, 2020.
11 Although lopinavir/ritonavir (LPN/r), an antiretroviral agent used to treat human immunodeficiency virus (HIV) infection, was initially thought to inhibit the 3-chymotrypsin-like protease of SARS-CoV-2,
12 guidelines released on April 21, 2020, recommended that it not be used as its benefits were unclear.
613
The purpose of this study was to determine whether Korean patients with COVID-19 were appropriately treated during the first year of the COVID-19 pandemic, in the absence of sufficient Korean medical guidelines.
METHODS
Data source and study population
A retrospective cohort was constructed using Nationwide claims registered in the database of the National Health Insurance System (NHIS) of Korea from January 2015 to December 2020. This database includes diagnoses, procedures, healthcare check-ups, and prescription records for all patients, anonymized according to confidentiality guidelines. Patients hospitalized between February 2020 and December 2020 for COVID-19 were screened using the diagnosis codes U071 and U072, which could be assigned only if confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR). This study included only hospitalized patients who were isolated and excluded patients who visited the outpatient clinic for complications of COVID-19 and patients who were isolated in community treatment centers because they were not expected to receive antiviral agents.
We extracted detailed data on each patient’s demographic characteristics, medications, treatments received, and hospitals in which they were treated. Patient factors, including age, sex, underlying diseases, and type of insurance during the month of hospitalization, were collected. Underlying diseases, including hypertension, diabetes mellitus, dyslipidemia, myocardial infarction, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, chronic liver disease, and malignancy, diagnosed before the COVID-19 admission, were defined as comorbidities. Oxygen requirement was regarded as a proxy indicator of COVID-19 severity. Hospital information included the type of hospital (tertiary referral, general, or primary/nursing and whether the hospital was private or public), the number of beds in the hospital, and the regional location. Hospital regions were categorized according to the administrative regions of Korea: Seoul, the greater Seoul area, other metropolitan cities, and rural areas.
Prescription of antiviral candidates
Drug prescription records were collected and reviewed to determine whether these patients were prescribed drugs with expected antiviral effects during their COVID-19 episodes. A patient transferred to another hospital during the study period was regarded as a separate COVID-19 hospitalization, with the prescription records separately reviewed in the second hospital. Antiviral agents suggested in previous studies were screened,
1415 and drugs prescribed during a relatively high percentage of hospitalization episodes were selected as target drugs. Based on these criteria, HCQ and LPN/r were selected as candidate antiviral drugs for COVID-19 treatment.
A prescription of HCQ for COVID-19 treatment was included when it was administered to hospitalized patients diagnosed with COVID-19, not to patients diagnosed with malaria or rheumatologic diseases such as rheumatoid arthritis and systemic lupus erythematosus, and not coprescribed with primaquine. Similarly, a prescription of LPN/r for COVID-19 treatment was included when it was administered to hospitalized patients diagnosed with COVID-19 isolation, not to patients diagnosed with HIV and not prescribed concurrently to patients receiving other antiretroviral agents.
An antiviral prescription was considered ineffective when that drug was prescribed after guidelines recommended that it not be used to treat COVID-19. NIH guidelines recommended against using LPN/r on April 21, 2020, and against using HCQ on June 11, 2020. This study assumed that approximately 1 month would be required for the revised guidelines to be disseminated nationally. Thus, in accordance with the updated NIH COVID-19 treatment guidelines, prescriptions of LPN/r and HCQ after July 2020 were regarded as ineffective.
Statistical analysis
Baseline characteristics, including patient and hospital factors relative to the total number of COVID-19 admission episodes, were descriptively analyzed in groups that did and did not receive each antiviral and in groups before and after July 2020. Categorical variables were presented as frequencies with percentages and continuous variables as mean ± standard deviation (SD). Prescriptions of HCQ and LPN/r were also analyzed monthly, and the monthly prescription frequencies of HCQ, LPN/r, and steroids were compared in patients who did and did not receive oxygen therapy. Hospital factors associated with HCQ and LPN/r prescriptions were determined by comparing the ratio of each antiviral agent prescription relative to the total numbers of COVID-19 admissions before and after July 2020.
Risk factors associated with the prescription of ineffective antiviral agents were identified by univariate and multivariate logistic regression analyses. Patients prescribed ineffective antivirals were compared with patients not administered antiviral agents during the same period. Patient factors, including age, sex, type of insurance, comorbidities, and disease severity, and hospital factors, including type of hospital, number of beds, and region location, were included in the logistic analyses. The hospital regions were categorized into three groups, namely, low, middle, and high, based on the frequency of prescribed antivirals before July 2020. All statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA).
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (IRB No. 2112-064-1281), which waived the requirement for informed consent due to the retrospective nature of this study and the anonymization of all clinical data.
RESULTS
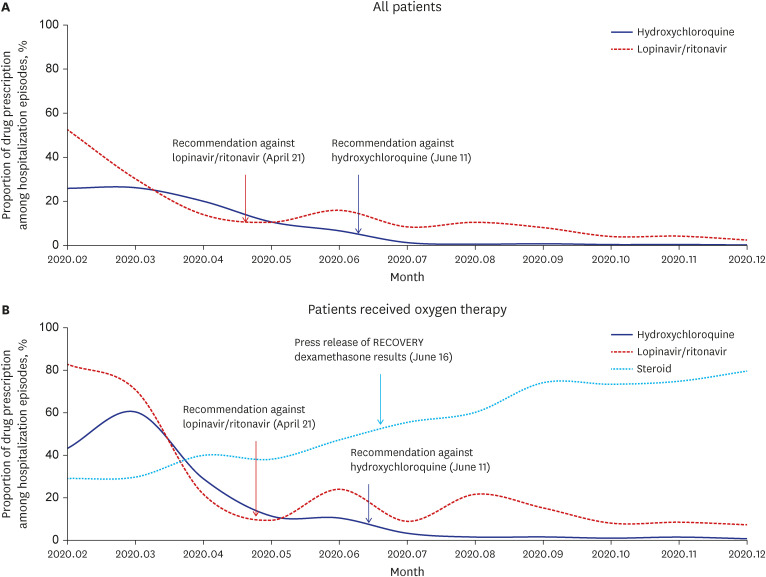
A total of 114,533 individuals were diagnosed with COVID-19 in 2020, with 64,566 hospital admission episodes for COVID-19 during this period. These 64,566 hospital admission episodes included 15,723 episodes before and 48,843 after guideline revisions in July 2020. Of the 15,723 COVID-19 admission episodes before guideline revisions, 3,312 (21.1%) included prescriptions of HCQ, and 4,183 (26.6%) included prescriptions of LPN/r. Of the 48,843 admission episodes after guideline revisions, 182 (0.4%) and 2,258 (4.6%) included prescriptions of ineffective HCQ and LPN/r, respectively. Prescriptions of HCQ consistently decreased after guideline revision, being rarely prescribed (0.2–0.7% of total admissions) after July 2020. By contrast, although prescription of LPN/r decreased after guideline revision, it continued to be prescribed steadily in small amounts, to 7.3–21.6% of total admission, until December 2020. This trend was also observed in patients receiving oxygen therapy, but the LPN/r prescription rates were relatively higher in the overall patient population. Corticosteroid prescription also increased steadily, being prescribed to around 80% of patients hospitalized since September 2020 (
Fig. 1). In patients not receiving oxygen therapy, the prescriptions of HCQ and LPN/r were gradually reduced, and corticosteroids were prescribed to only 1.8–6.8% of patients throughout the study period (
Supplementary Fig. 1).
Fig. 1
Chronological presentation of the prescription of candidate antiviral agents and corticosteroids for coronavirus disease 2019 treatment in Korea according to disease severity. (A) All patients, (B) patients who received oxygen therapy.
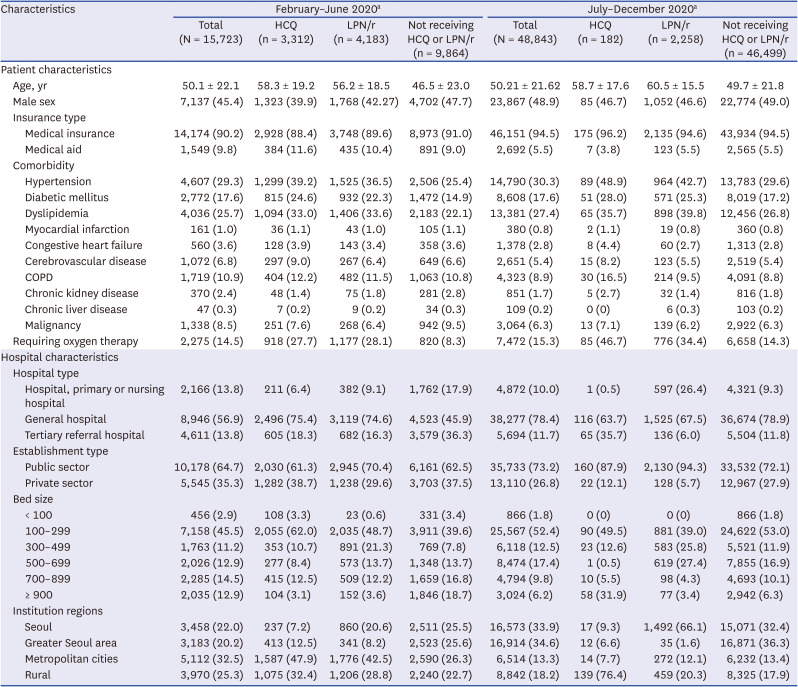
Patient factors associated with antivirals prescribed during hospitalization episodes before and after the guideline revision are presented in
Table 1. Patients treated with antiviral agents were older than those not treated with antiviral agents. Types of insurance were similar in these two groups. The percentages with underlying diseases, including hypertension, diabetes, dyslipidemia, and chronic obstructive lung disease, were higher in patients who did than did not receive antiviral agents, both before and after guideline revision. The percentage receiving oxygen therapy was higher in patients who did than did not receive candidate antivirals, with these percentages being higher after than before the guideline revision, increasing from 27.7% to 46.7% in patients administered HCQ and from 28.1% to 34.4% in patients administered LPN/r.
Table 1
Baseline characteristics of COVID-19 patients treated in hospitals between February 2020 and December 2020 in Korea
|
Characteristics |
February–June 2020a
|
July–December 2020a
|
|
Total (N = 15,723) |
HCQ (n = 3,312) |
LPN/r (n = 4,183) |
Not receiving HCQ or LPN/r (n = 9,864) |
Total (N = 48,843) |
HCQ (n = 182) |
LPN/r (n = 2,258) |
Not receiving HCQ or LPN/r (n = 46,499) |
|
Patient characteristics |
|
|
|
|
|
|
|
|
|
Age, yr |
50.1 ± 22.1 |
58.3 ± 19.2 |
56.2 ± 18.5 |
46.5 ± 23.0 |
50.21 ± 21.62 |
58.7 ± 17.6 |
60.5 ± 15.5 |
49.7 ± 21.8 |
|
Male sex |
7,137 (45.4) |
1,323 (39.9) |
1,768 (42.27) |
4,702 (47.7) |
23,867 (48.9) |
85 (46.7) |
1,052 (46.6) |
22,774 (49.0) |
|
Insurance type |
|
|
|
|
|
|
|
|
|
|
Medical insurance |
14,174 (90.2) |
2,928 (88.4) |
3,748 (89.6) |
8,973 (91.0) |
46,151 (94.5) |
175 (96.2) |
2,135 (94.6) |
43,934 (94.5) |
|
|
Medical aid |
1,549 (9.8) |
384 (11.6) |
435 (10.4) |
891 (9.0) |
2,692 (5.5) |
7 (3.8) |
123 (5.5) |
2,565 (5.5) |
|
Comorbidity |
|
|
|
|
|
|
|
|
|
|
Hypertension |
4,607 (29.3) |
1,299 (39.2) |
1,525 (36.5) |
2,506 (25.4) |
14,790 (30.3) |
89 (48.9) |
964 (42.7) |
13,783 (29.6) |
|
|
Diabetic mellitus |
2,772 (17.6) |
815 (24.6) |
932 (22.3) |
1,472 (14.9) |
8,608 (17.6) |
51 (28.0) |
571 (25.3) |
8,019 (17.2) |
|
|
Dyslipidemia |
4,036 (25.7) |
1,094 (33.0) |
1,406 (33.6) |
2,183 (22.1) |
13,381 (27.4) |
65 (35.7) |
898 (39.8) |
12,456 (26.8) |
|
|
Myocardial infarction |
161 (1.0) |
36 (1.1) |
43 (1.0) |
105 (1.1) |
380 (0.8) |
2 (1.1) |
19 (0.8) |
360 (0.8) |
|
|
Congestive heart failure |
560 (3.6) |
128 (3.9) |
143 (3.4) |
358 (3.6) |
1,378 (2.8) |
8 (4.4) |
60 (2.7) |
1,313 (2.8) |
|
|
Cerebrovascular disease |
1,072 (6.8) |
297 (9.0) |
267 (6.4) |
649 (6.6) |
2,651 (5.4) |
15 (8.2) |
123 (5.5) |
2,519 (5.4) |
|
|
COPD |
1,719 (10.9) |
404 (12.2) |
482 (11.5) |
1,063 (10.8) |
4,323 (8.9) |
30 (16.5) |
214 (9.5) |
4,091 (8.8) |
|
|
Chronic kidney disease |
370 (2.4) |
48 (1.4) |
75 (1.8) |
281 (2.8) |
851 (1.7) |
5 (2.7) |
32 (1.4) |
816 (1.8) |
|
|
Chronic liver disease |
47 (0.3) |
7 (0.2) |
9 (0.2) |
34 (0.3) |
109 (0.2) |
0 (0) |
6 (0.3) |
103 (0.2) |
|
|
Malignancy |
1,338 (8.5) |
251 (7.6) |
268 (6.4) |
942 (9.5) |
3,064 (6.3) |
13 (7.1) |
139 (6.2) |
2,922 (6.3) |
|
Requiring oxygen therapy |
2,275 (14.5) |
918 (27.7) |
1,177 (28.1) |
820 (8.3) |
7,472 (15.3) |
85 (46.7) |
776 (34.4) |
6,658 (14.3) |
|
Hospital characteristics |
|
|
|
|
|
|
|
|
|
Hospital type |
|
|
|
|
|
|
|
|
|
|
Hospital, primary or nursing hospital |
2,166 (13.8) |
211 (6.4) |
382 (9.1) |
1,762 (17.9) |
4,872 (10.0) |
1 (0.5) |
597 (26.4) |
4,321 (9.3) |
|
|
General hospital |
8,946 (56.9) |
2,496 (75.4) |
3,119 (74.6) |
4,523 (45.9) |
38,277 (78.4) |
116 (63.7) |
1,525 (67.5) |
36,674 (78.9) |
|
|
Tertiary referral hospital |
4,611 (13.8) |
605 (18.3) |
682 (16.3) |
3,579 (36.3) |
5,694 (11.7) |
65 (35.7) |
136 (6.0) |
5,504 (11.8) |
|
Establishment type |
|
|
|
|
|
|
|
|
|
|
Public sector |
10,178 (64.7) |
2,030 (61.3) |
2,945 (70.4) |
6,161 (62.5) |
35,733 (73.2) |
160 (87.9) |
2,130 (94.3) |
33,532 (72.1) |
|
|
Private sector |
5,545 (35.3) |
1,282 (38.7) |
1,238 (29.6) |
3,703 (37.5) |
13,110 (26.8) |
22 (12.1) |
128 (5.7) |
12,967 (27.9) |
|
Bed size |
|
|
|
|
|
|
|
|
|
|
< 100 |
456 (2.9) |
108 (3.3) |
23 (0.6) |
331 (3.4) |
866 (1.8) |
0 (0) |
0 (0) |
866 (1.8) |
|
|
100–299 |
7,158 (45.5) |
2,055 (62.0) |
2,035 (48.7) |
3,911 (39.6) |
25,567 (52.4) |
90 (49.5) |
881 (39.0) |
24,622 (53.0) |
|
|
300–499 |
1,763 (11.2) |
353 (10.7) |
891 (21.3) |
769 (7.8) |
6,118 (12.5) |
23 (12.6) |
583 (25.8) |
5,521 (11.9) |
|
|
500–699 |
2,026 (12.9) |
277 (8.4) |
573 (13.7) |
1,348 (13.7) |
8,474 (17.4) |
1 (0.5) |
619 (27.4) |
7,855 (16.9) |
|
|
700–899 |
2,285 (14.5) |
415 (12.5) |
509 (12.2) |
1,659 (16.8) |
4,794 (9.8) |
10 (5.5) |
98 (4.3) |
4,693 (10.1) |
|
|
≥ 900 |
2,035 (12.9) |
104 (3.1) |
152 (3.6) |
1,846 (18.7) |
3,024 (6.2) |
58 (31.9) |
77 (3.4) |
2,942 (6.3) |
|
Institution regions |
|
|
|
|
|
|
|
|
|
|
Seoul |
3,458 (22.0) |
237 (7.2) |
860 (20.6) |
2,511 (25.5) |
16,573 (33.9) |
17 (9.3) |
1,492 (66.1) |
15,071 (32.4) |
|
|
Greater Seoul area |
3,183 (20.2) |
413 (12.5) |
341 (8.2) |
2,523 (25.6) |
16,914 (34.6) |
12 (6.6) |
35 (1.6) |
16,871 (36.3) |
|
|
Metropolitan cities |
5,112 (32.5) |
1,587 (47.9) |
1,776 (42.5) |
2,590 (26.3) |
6,514 (13.3) |
14 (7.7) |
272 (12.1) |
6,232 (13.4) |
|
|
Rural |
3,970 (25.3) |
1,075 (32.4) |
1,206 (28.8) |
2,240 (22.7) |
8,842 (18.2) |
139 (76.4) |
459 (20.3) |
8,325 (17.9) |
Prior to July 2020, the rate of HCQ prescriptions was highest in patients hospitalized in general hospitals (27.9%), followed by tertiary hospitals (13.5%) and primary/nursing hospitals (9.1%). The rate of LPN/r prescriptions was also highest in general hospitals (34.9%), followed by primary/nursing hospitals (16.6%) and tertiary hospitals (15.3%). After July 2020, the rates of LPN/r prescriptions in general and tertiary hospitals declined drastically to 4.0% and 2.4%, respectively, whereas the rate in primary hospitals declined moderately to 12.3%. After July 2020, the rates of HCQ prescriptions had declined to below 1.2% in all hospital types. Prior to July 2020, the proportions of patients prescribed HCQ and LPN/r were 19.9% and 28.9%, respectively, in public hospitals, and 23.1% and 22.3%, respectively, in private hospitals. After July 2020, the proportions of patients prescribed HCQ and LPN/r in private hospitals declined to below 1%, while the proportion prescribed LPN/r in public hospitals was 6.0%. The proportions of inpatients prescribed HCQ and LPN/r varied markedly based on bed size and hospital location (
Supplementary Fig. 2).
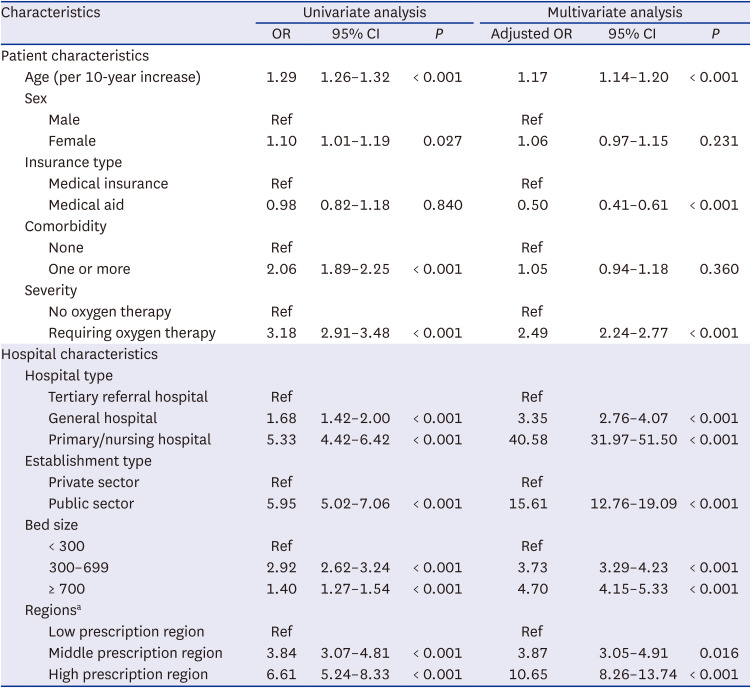
Univariate logistic regression analyses showed that patient age (odds ratio [OR] per 10-year increase, 1.29; 95% confidential interval [CI], 1.26–1.32;
P < 0.001) and one or more comorbidities (OR, 2.06; 95% CI, 1.89–2.25;
P < 0.001) were associated with a modestly increased risk of being prescribed an ineffective antiviral agent. In addition, severe disease requiring oxygen therapy was associated with an increased risk of being prescribed an ineffective antiviral agent (OR, 3.18; 95% CI, 2.91–3.48;
P < 0.001), as did hospitalization in a primary/nursing hospital (OR, 5.33; 95% CI, 4.42–6.42;
P < 0.001) and in a public sector hospital (OR, 5.95; 95% CI, 5.02–7.06;
P < 0.001). Geographic regions with a high prescription rate before the guideline revision were significantly associated with an increased risk of an ineffective antiviral agent prescription after the guideline revision (OR, 6.61; 95% CI, 5.24–8.33;
P < 0.001). These trends, except for comorbidity, were maintained in the multivariate analysis (
Table 2).
Table 2
Univariate and multivariate analyses of factors significantly associated with the prescription of ineffective antiviral drugs for COVID-19 between July 2020 and December 2020 in Korea
|
Characteristics |
Univariate analysis |
Multivariate analysis |
|
OR |
95% CI |
P
|
Adjusted OR |
95% CI |
P
|
|
Patient characteristics |
|
|
|
|
|
|
|
Age (per 10-year increase) |
1.29 |
1.26–1.32 |
< 0.001 |
1.17 |
1.14–1.20 |
< 0.001 |
|
Sex |
|
|
|
|
|
|
|
|
Male |
Ref |
|
|
Ref |
|
|
|
|
Female |
1.10 |
1.01–1.19 |
0.027 |
1.06 |
0.97–1.15 |
0.231 |
|
Insurance type |
|
|
|
|
|
|
|
|
Medical insurance |
Ref |
|
|
Ref |
|
|
|
|
Medical aid |
0.98 |
0.82–1.18 |
0.840 |
0.50 |
0.41–0.61 |
< 0.001 |
|
Comorbidity |
|
|
|
|
|
|
|
|
None |
Ref |
|
|
Ref |
|
|
|
|
One or more |
2.06 |
1.89–2.25 |
< 0.001 |
1.05 |
0.94–1.18 |
0.360 |
|
Severity |
|
|
|
|
|
|
|
|
No oxygen therapy |
Ref |
|
|
Ref |
|
|
|
|
Requiring oxygen therapy |
3.18 |
2.91–3.48 |
< 0.001 |
2.49 |
2.24–2.77 |
< 0.001 |
|
Hospital characteristics |
|
|
|
|
|
|
|
Hospital type |
|
|
|
|
|
|
|
|
Tertiary referral hospital |
Ref |
|
|
Ref |
|
|
|
|
General hospital |
1.68 |
1.42–2.00 |
< 0.001 |
3.35 |
2.76–4.07 |
< 0.001 |
|
|
Primary/nursing hospital |
5.33 |
4.42–6.42 |
< 0.001 |
40.58 |
31.97–51.50 |
< 0.001 |
|
Establishment type |
|
|
|
|
|
|
|
|
Private sector |
Ref |
|
|
Ref |
|
|
|
|
Public sector |
5.95 |
5.02–7.06 |
< 0.001 |
15.61 |
12.76–19.09 |
< 0.001 |
|
Bed size |
|
|
|
|
|
|
|
|
< 300 |
Ref |
|
|
Ref |
|
|
|
|
300–699 |
2.92 |
2.62–3.24 |
< 0.001 |
3.73 |
3.29–4.23 |
< 0.001 |
|
|
≥ 700 |
1.40 |
1.27–1.54 |
< 0.001 |
4.70 |
4.15–5.33 |
< 0.001 |
|
Regionsa
|
|
|
|
|
|
|
|
|
Low prescription region |
Ref |
|
|
Ref |
|
|
|
|
Middle prescription region |
3.84 |
3.07–4.81 |
< 0.001 |
3.87 |
3.05–4.91 |
0.016 |
|
|
High prescription region |
6.61 |
5.24–8.33 |
< 0.001 |
10.65 |
8.26–13.74 |
< 0.001 |
DISCUSSION
The COVID-19 pandemic was a type of infectious disease disaster, making it necessary to determine whether the response to this disaster was appropriate, thereby enabling preparation for future pandemics. Because a large number of patients with this novel infectious disease visited medical institutions, many of them required treatment by physicians who were not infectious disease specialists.
The results of this study showed that ineffective antiviral agents were prescribed to a substantial number of patients, despite revised guidelines recommending these agents not be used. HCQ prescriptions decreased markedly after the U.S. FDA withdrew emergency use authorization. By contrast, LPN/r was prescribed to more than 2,000 patients more than 2 months after the NIH recommended on April 17, 2020, that it not be used. Because remdesivir became available in August 2020 to treat patients in Korea with severe COVID-19, unnecessary prescriptions of LPN/r could have been further reduced.
Both hospital-related and patient-related factors were associated with prescription of ineffective antiviral agents. Hospital factors, however, showed a stronger association, with public sector compared with private sector hospitals showing the highest association with ineffective antiviral use. Many studies have evaluated the appropriate roles and balance of the private and public sectors in providing healthcare services. A systematic review of middle to low income countries reported that the private sector performed better in relation to drug supply, responsiveness, and effort.
16 However, a follow-up study that broadened the evaluation categories found that the private sector was neither more efficient nor more effective than the public sector.
17 The present results provide evidence that awareness of changes in COVID-19 treatment guidelines with antiviral agents occurred earlier in the private than in the public sector.
The use of ineffective antiviral agents was more frequent in primary/nursing hospitals, perhaps because small hospitals are less likely to have an infectious disease specialist on-site than larger hospitals, reducing their likelihood of exposure to appropriate antiviral treatment. Moreover, because the procedure for obtaining remdesivir in Korea was complicated in 2020, an ineffective antiviral agent may have been chosen over remdesivir by hospitals with a severe clinical burden. In addition, ineffective antiviral drugs may have been administered in medical surge situations as a shortage of beds in large hospitals may have made patient transfer difficult. Even in these situations, however, the ineffectiveness of these antiviral agents precluded their improper use, thus making it necessary to educate clinicians about updated treatment guidelines, similar to evaluating and improving physician adherence to antibiotic treatment guidelines. Communication and education are important strategies for both antibiotic and antiviral stewardship.
Although corticosteroids are not categorized as antiviral drugs, the patterns of corticosteroid prescription for severe COVID-19 were evaluated. Because corticosteroids inhibit the innate immune system, their use in viral infection has been generally discouraged due to the concerns about viral proliferation. Indeed, guidelines recommended that corticosteroids should be avoided during the early period of the COVID-19 pandemic.
1819 After the RECOVERY trial demonstrated the survival benefit of dexamethasone in patients with severe COVID-19,
20 many treatment guidelines, including those of the NIH and WHO, recommended that dexamethasone be administered to COVID-19 patients requiring oxygen supplementation.
2122 About 50% of COVID-19 patients receiving oxygen treatment in June 2020 were also treated with corticosteroids, with this rate gradually increasing to 80% in December 2020, suggesting that these guidelines were disseminated relatively quickly.
Despite research on emerging infectious diseases, the development and dissemination of clinical guidelines remain a problem. Although drug prescription during the first year of the COVID-19 pandemic in the United States was analyzed, that study did not evaluate the dissemination of treatment recommendations.
23 The excess clinical burden on infectious disease specialists during the pandemic may have resulted in a lack of guidance or education.
24
A German qualitative study on the reasons for not following guidelines suggested lack of applicability or lack of evidence (68% of key recommendations), environmental factors such as organizational constraints (52%), lack of knowledge regarding guideline recommendations (46%), and guideline factors such as unclear or ambiguous guideline recommendations (43%).
25 Physician adherence to guidelines may be improved by methods such as small group meetings, audit and feedback, organizational interventions, and use of local opinion leaders.
26 Guidelines should be ‘customer-driven’ for physicians, suggesting the need for the timely availability of Korean guidelines.
This study had several limitations. First, it did not evaluate the antiviral prescription patterns by individual doctors or medical specialty because information about prescribing physicians was unavailable. Regardless of the specialty of the doctor in charge, however, doctors in larger hospitals of more than 300 beds were likely exposed to infectious disease specialists. Second, the administration of government-supplied remdesivir was not included in the NHIS database; thus, this study was unable to assess the effect of remdesivir supply on antiviral drug use patterns. Third, as the study used the secondary claims data of NHIS, detailed clinical information on the enrolled patients, including laboratory measurements, symptoms, and disease progression, could not be confirmed, and their effect on the prescription of ineffective antiviral drugs could not be investigated.
In conclusion, a substantial number of patients hospitalized for COVID-19 during the first year of the pandemic in Korea were prescribed ineffective antiviral agents. Preparative strategies for future epidemics should include the dissemination of optimal treatment guidelines to front-line facilities, particularly small-sized, public sector hospitals.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download