1. Caudle AS, Cupp JA, Kuerer HM. Management of axillary disease. Surg Oncol Clin N Am. 2014; 23(3):473–486. PMID:
24882346.

2. Burak WE Jr, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002; 183(1):23–27. PMID:
11869698.

3. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23(30):7703–7720. PMID:
16157938.

4. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011; 305(6):569–575. PMID:
21304082.

5. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017; 318(10):918–926. PMID:
28898379.

6. van Roozendaal LM, Vane MLG, van Dalen T, van der Hage JA, Strobbe LJA, Boersma LJ, et al. Clinically node negative breast cancer patients undergoing breast conserving therapy, sentinel lymph node procedure versus follow-up: a Dutch randomized controlled multicentre trial (BOOG 2013-08). BMC Cancer. 2017; 17(1):459. PMID:
28668073.

7. Gentilini O, Botteri E, Dadda P, Sangalli C, Boccardo C, Peradze N, et al. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur J Surg Oncol. 2016; 42(5):685–689. PMID:
26899941.

8. Reimer T, Stachs A, Nekljudova V, Loibl S, Hartmann S, Wolter K, et al. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (c/it1-2) in the context of breast conserving therapy: first results following commencement of the Intergroup-Sentinel-Mamma (INSEMA) trial. Geburtshilfe Frauenheilkd. 2017; 77(2):149–157. PMID:
28331237.

9. Tucker NS, Cyr AE, Ademuyiwa FO, Tabchy A, George K, Sharma PK, et al. Axillary ultrasound accurately excludes clinically significant lymph node disease in patients with early stage breast cancer. Ann Surg. 2016; 264(6):1098–1102. PMID:
26779976.

10. Chang JM, Leung JW, Moy L, Ha SM, Moon WK. Axillary nodal evaluation in breast cancer: state of the art. Radiology. 2020; 295(3):500–515. PMID:
32315268.

11. Kuijs VJ, Moossdorff M, Schipper RJ, Beets-Tan RG, Heuts EM, Keymeulen KB, et al. The role of MRI in axillary lymph node imaging in breast cancer patients: a systematic review. Insights Imaging. 2015; 6(2):203–215. PMID:
25800994.

12. van Nijnatten TJ, Ploumen EH, Schipper RJ, Goorts B, Andriessen EH, Vanwetswinkel S, et al. Routine use of standard breast MRI compared to axillary ultrasound for differentiating between no, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur J Radiol. 2016; 85(12):2288–2294. PMID:
27842679.

13. Abe H, Schacht D, Kulkarni K, Shimauchi A, Yamaguchi K, Sennett CA, et al. Accuracy of axillary lymph node staging in breast cancer patients: an observer-performance study comparison of MRI and ultrasound. Acad Radiol. 2013; 20(11):1399–1404. PMID:
24119352.
14. Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005; 91(2):113–119. PMID:
15868438.

15. Degnim AC, Reynolds C, Pantvaidya G, Zakaria S, Hoskin T, Barnes S, et al. Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg. 2005; 190(4):543–550. PMID:
16164917.

16. Bae MS, Shin SU, Song SE, Ryu HS, Han W, Moon WK. Association between US features of primary tumor and axillary lymph node metastasis in patients with clinical T1-T2N0 breast cancer. Acta Radiol. 2018; 59(4):402–408. PMID:
28748712.

17. Yi A, Moon WK, Cho N, Chang JM, Bae MS, Kim SJ, et al. Association of tumour stiffness on sonoelastography with axillary nodal status in T1 breast carcinoma patients. Eur Radiol. 2013; 23(11):2979–2987. PMID:
23783782.

18. Zhou LQ, Wu XL, Huang SY, Wu GG, Ye HR, Wei Q, et al. Lymph node metastasis prediction from primary breast cancer US images using deep learning. Radiology. 2020; 294(1):19–28. PMID:
31746687.

19. Ha R, Chang P, Karcich J, Mutasa S, Fardanesh R, Wynn RT, et al. Axillary lymph node evaluation utilizing convolutional neural networks using MRI dataset. J Digit Imaging. 2018; 31(6):851–856. PMID:
29696472.

20. Han L, Zhu Y, Liu Z, Yu T, He C, Jiang W, et al. Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol. 2019; 29(7):3820–3829. PMID:
30701328.

21. Hortobagyi GN, Connolly JL, D'Orsi CJ, Edge SB, Mittendorf EA, Rugo HS. Breast. American joint committee on cancer. Amin MB, Edge SB, editors. AJCC cancer staging manual. 8th ed. Chicago IL: Springer;2017. p. 589–628.
23. Ecanow JS, Abe H, Newstead GM, Ecanow DB, Jeske JM. Axillary staging of breast cancer: what the radiologist should know. Radiographics. 2013; 33(6):1589–1612. PMID:
24108553.

24. Baltzer PA, Dietzel M, Burmeister HP, Zoubi R, Gajda M, Camara O, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol. 2011; 196(5):W641-7. PMID:
21512057.

25. American college of radiology. ACR BI-RADS Atlas: breast imaging reporting and data system. 5th ed. Reston, VA: American college of radiology;2013.
26. Sacre RA. Clinical evaluation of axillar lymph nodes compared to surgical and pathological findings. Eur J Surg Oncol. 1986; 12(2):169–173. PMID:
3709822.
27. Keelan S, Heeney A, Downey E, Hegarty A, Roche T, Power C, et al. Breast cancer patients with a negative axillary ultrasound may have clinically significant nodal metastasis. Breast Cancer Res Treat. 2021; 187(2):303–310. PMID:
33837870.

28. Neal CH, Daly CP, Nees AV, Helvie MA. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010; 257(2):335–341. PMID:
20807849.

29. Boughey JC, Alvarado MD, Lancaster RB, Fraser Symmans W, Mukhtar R, Wong JM, et al. Surgical standards for management of the axilla in breast cancer clinical trials with pathological complete response endpoint. NPJ Breast Cancer. 2018; 4(1):26. PMID:
30131975.

30. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017; 3(4):549–555. PMID:
27918753.

31. Byon JH, Park YV, Yoon JH, Moon HJ, Kim EK, Kim MJ, et al. Added value of MRI for Invasive breast cancer including the Entire axilla for evaluation of high-level or advanced axillary lymph node metastasis in the post-ACOSOG Z0011 trial era. Radiology. 2021; 300(1):46–54. PMID:
33904772.

32. Zhang Y, Li J, Fan Y, Li X, Qiu J, Zhu M, et al. Risk factors for axillary lymph node metastases in clinical stage T1-2N0M0 breast cancer patients. Medicine (Baltimore). 2019; 98(40):e17481. PMID:
31577783.

33. Liang Y, Benakanakere I, Besch-Williford C, Hyder RS, Ellersieck MR, Hyder SM. Synthetic progestins induce growth and metastasis of BT-474 human breast cancer xenografts in nude mice. Menopause. 2010; 17(5):1040–1047. PMID:
20461021.

34. Rosati R, Oppat K, Huang Y, Kim S, Ratnam M. Clinical association of progesterone receptor isoform a with breast cancer metastasis consistent with its unique mechanistic role in preclinical models. BMC Cancer. 2020; 20(1):512. PMID:
32493230.

35. Chang JM, Shin HJ, Choi JS, Shin SU, Choi BH, Kim MJ, et al. Imaging protocol and criteria for evaluation of axillary lymph nodes in the NAUTILUS trial. J Breast Cancer. 2021; 24(6):554–560. PMID:
34877830.

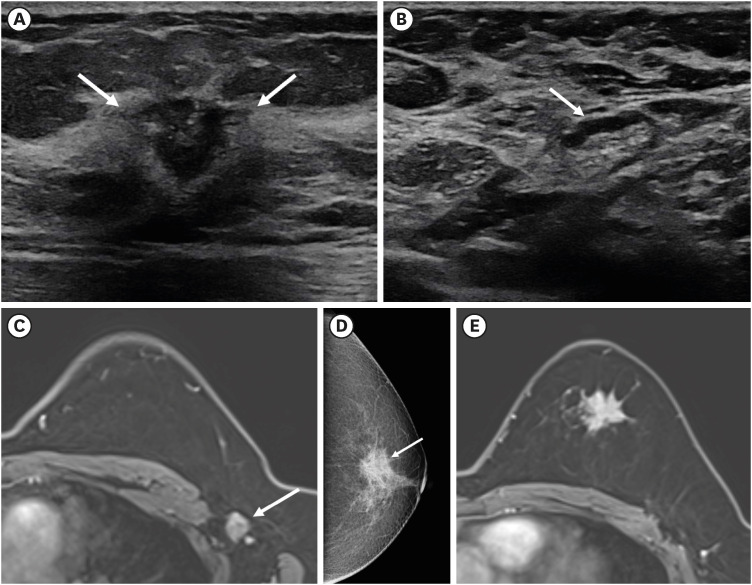
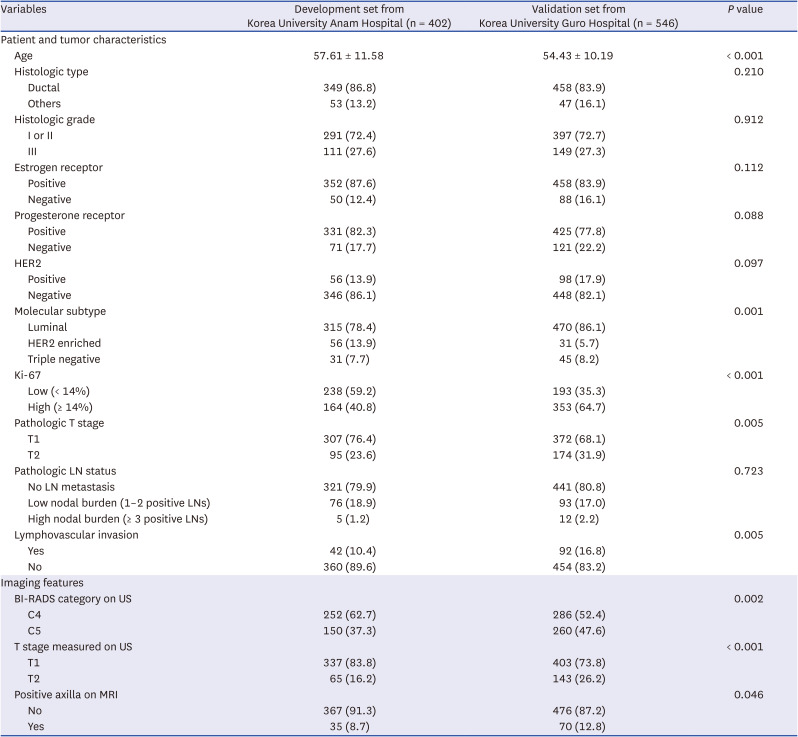
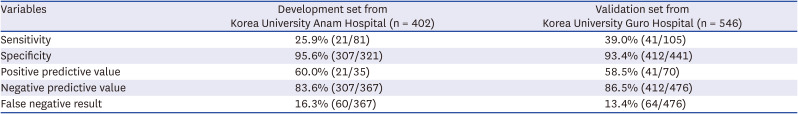
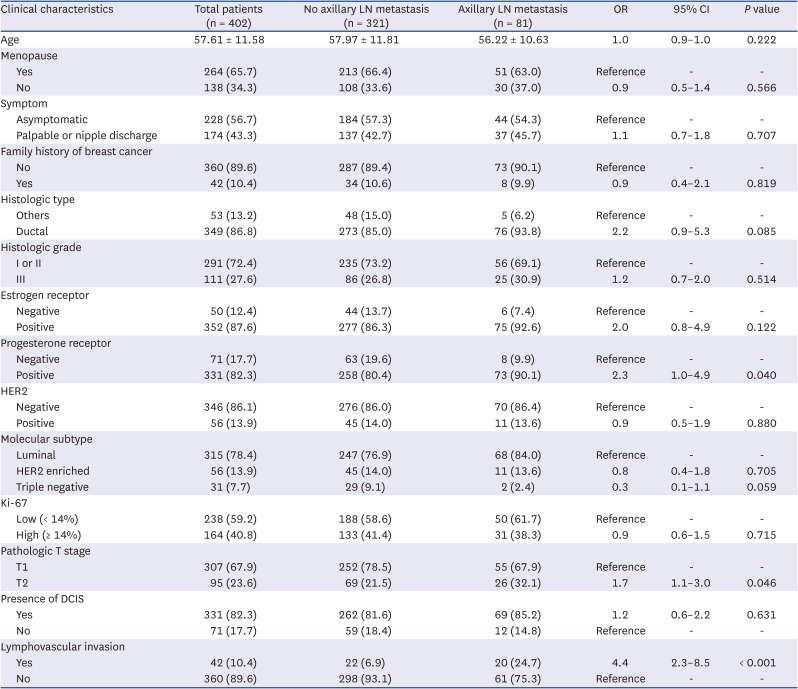




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download