This article has been corrected. See "The coronavirus disease 2019 infection in pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis" in Volume 66 on page 587.
Abstract
The coronavirus disease 2019 (COVID-19) outbreak which started in December 2019 rapidly developed into a global health concern. Pregnant women are susceptible to respiratory infections and can experience adverse outcomes. This systematic review and meta-analysis compared pregnancy outcomes according to COVID-19 disease status. The MEDLINE, EMBASE, and Cochrane Library databases were searched for relevant articles published between December 1, 2019, and October 19, 2022. Main inclusion criterion was any population-based, cross-sectional, cohort, or case-control study that assessed pregnancy outcomes in women with or without laboratory-confirmed COVID-19. Sixty-nine studies including 1,606,543 pregnant women (39,716 [2.4%] diagnosed with COVID-19) were retrieved. COVID-19-infected pregnant women had a higher risk of preterm birth (odds ratio [OR], 1.59; 95% confidence interval [CI], 1.42–1.78), preeclampsia (OR, 1.41; 95% CI, 1.30–1.53), low birth weight (OR, 1.52; 95% CI, 1.30–1.79), cesarean delivery (OR, 1.20; 95% CI, 1.10–1.30), stillbirth (OR, 1.71; 95% CI, 1.39–2.10), fetal distress (OR, 2.49; 95% CI, 1.54–4.03), neonatal intensive care unit admission (OR, 2.33; 95% CI, 1.72–3.16), perinatal mortality (OR, 1.96; 95% CI, 1.15–3.34), and maternal mortality (OR, 6.15; 95% CI, 3.74–10.10). There were no significant differences in total miscarriage, preterm premature rupture of membranes, postpartum hemorrhage, cholestasis, or chorioamnionitis according to infection. This review demonstrates that COVID-19 infection during pregnancy can lead to adverse pregnancy outcomes. This information could aid researchers and clinicians in preparing for another pandemic caused by newly discovered respiratory viruses. The findings of this study may assist with evidence-based counseling and help clinicians manage pregnant women with COVID-19.
Coronavirus disease 2019 (COVID-19) is a viral respiratory disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It has been a global threat to public health owing to its high mortality and morbidity rates since the first outbreak in Wuhan, China, in December 2019. According to the World Health Organization, as of November 30, 2022, there were 639,132,486 confirmed cases of COVID-19 globally, including 6,614,082 deaths.
The experience of patients infected with COVID-19 varies significantly, ranging from asymptomatic to acute respiratory distress syndrome accompanied by high fever and severe respiratory symptoms, similar to those of the Middle East respiratory syndrome (MERS) and the SARS outbreak in 2003. Pregnant women undergo immunological and physiological changes that prevent fetal allograft rejection. These changes also increase their susceptibility to viral respiratory infections. The SARS outbreak in 2003 and MERS were associated with high fatality rates, with one-third of the infected pregnant women dying.
During the pandemic, pregnant women experienced considerable fear of the respiratory virus, besides anxiety regarding outcomes because it was unknown how the new viral outbreaks would affect pregnancy and neonatal prognosis, and vaccine safety has not been verified. Previous studies have reported increased rates of miscarriage, intrauterine growth retardation (IUGR), premature delivery, and fetal death during MERS. Furthermore, viral respiratory infections are a leading cause of maternal mortality [1,2].
The pregnancy and neonatal outcomes associated with COVID-19 are inconsistent and diverse. Some studies have indicated that SARS-CoV-2 infection is not associated with adverse pregnancy outcomes [3,4]. Large-scale data analyses have stressed the urgency of establishing strategies to help clinicians manage pregnancies and ensure safe maternal and childcare deliveries. This systematic review and meta-analysis were based on a thorough review of the existing literature. We investigated the association between COVID-19 infection during pregnancy and adverse pregnancy outcome to aid clinicians and researchers in managing pregnant women infected with COVID-19 currently and contribute to preparations for future respiratory virus outbreaks.
We searched the literature to identify articles that compared obstetrical and neonatal outcomes between COVID-19-infected and non-infected mothers. This systematic review and meta-analysis were conducted using the PROSPERO protocol (CRD42023402238). We performed a systematic online search of the MEDLINE, EMBASE, and Cochrane Library databases for relevant articles published until October 19, 2022. The databases were searched using a combination of Medical Subject Headings and text terms related to COVID-19 and pregnancy outcomes. The detailed search strategy used for each database is presented in Supplementary Table 1. Two reviewers (M.A.K. and Y.J.) independently selected studies. They first performed title and abstract screening and then assessed the full texts of the selected studies for eligibility according to the inclusion criteria. They cross-checked previously published review articles to identify additional studies that were missed during our online searches. Discrepancies were resolved by consensus-based discussions.
We selected full-text articles that reported the impact of COVID-19 on obstetrical and neonatal outcomes by comparing infected and non-infected mothers and those that met the inclusion criteria as follows. 1) A population-based, cross-sectional, cohort, or case-control study design. 2) Inclusion of laboratory-confirmed non-infected pregnant women or pregnant women before the pandemic as a control group. 3) The study population included pregnant women with laboratory-confirmed COVID-19 infection at any stage of gestation, or pregnant women with assigned codes for COVID-19 based on the International classification of disease 10th version and related health problems. 4) Results included pregnancy or neonatal outcomes according to the COVID-19 infection status.
Outcomes, such as miscarriage, preterm birth, preeclampsia, cesarean section, intrauterine fetal death (IUFD) or stillbirth, preterm premature rupture of membranes (PPROM), placental abruption, placenta previa, postpartum hemorrhage, and maternal mortality were assessed as obstetric outcomes. IUGR, small for gestational age (SGA), low birth weight (LBW), neonatal intensive care unit (NICU) admission, and perinatal mortality were assessed as neonatal outcomes. Studies that met the following criteria were excluded: case reports, case series, review articles, editorials or letters to the editor, conference abstracts, lack of a control group, publications other than English, and inadequate information for data extraction.
We reviewed the full-text articles for complete data extraction and clarity if the details were not explicitly mentioned in the abstracts. The information extracted from each study included the first author’s name, publication year, journal name, study design, country, study period, inclusion and exclusion criteria, and the number of women included in the case and control groups.
The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included studies. The NOS evaluates studies from three perspectives: study group selection, group comparability, and exposure or outcome of interest for case-control or cohort studies. According to the NOS, each study was awarded a maximum of nine points with scores for various questions in each domain. Studies were considered of high quality if they had a NOS score ≥6.
This systematic review and meta-analysis were conducted according to the protocols established in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the Cochrane handbook for systematic reviews. We used a random-effects model to calculate the pooled odds ratios (ORs) and 95% confidence intervals (CIs) for adverse pregnancy outcomes experienced by women with or without COVID-19 infection. Heterogeneity was assessed using the I2 statistic; values of >25%, >50%, and >75% were considered evidence of low, moderate, and considerable statistical heterogeneity, respectively. A sensitivity analysis was conducted to evaluate the influence of individual studies on the meta-analysis by restricting the studies individually and assessing the effect on the main summary estimate. Significance levels were set at P<0.05 if the 95% CI did not include 1. All statistical analyses were performed using RevMan (version 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
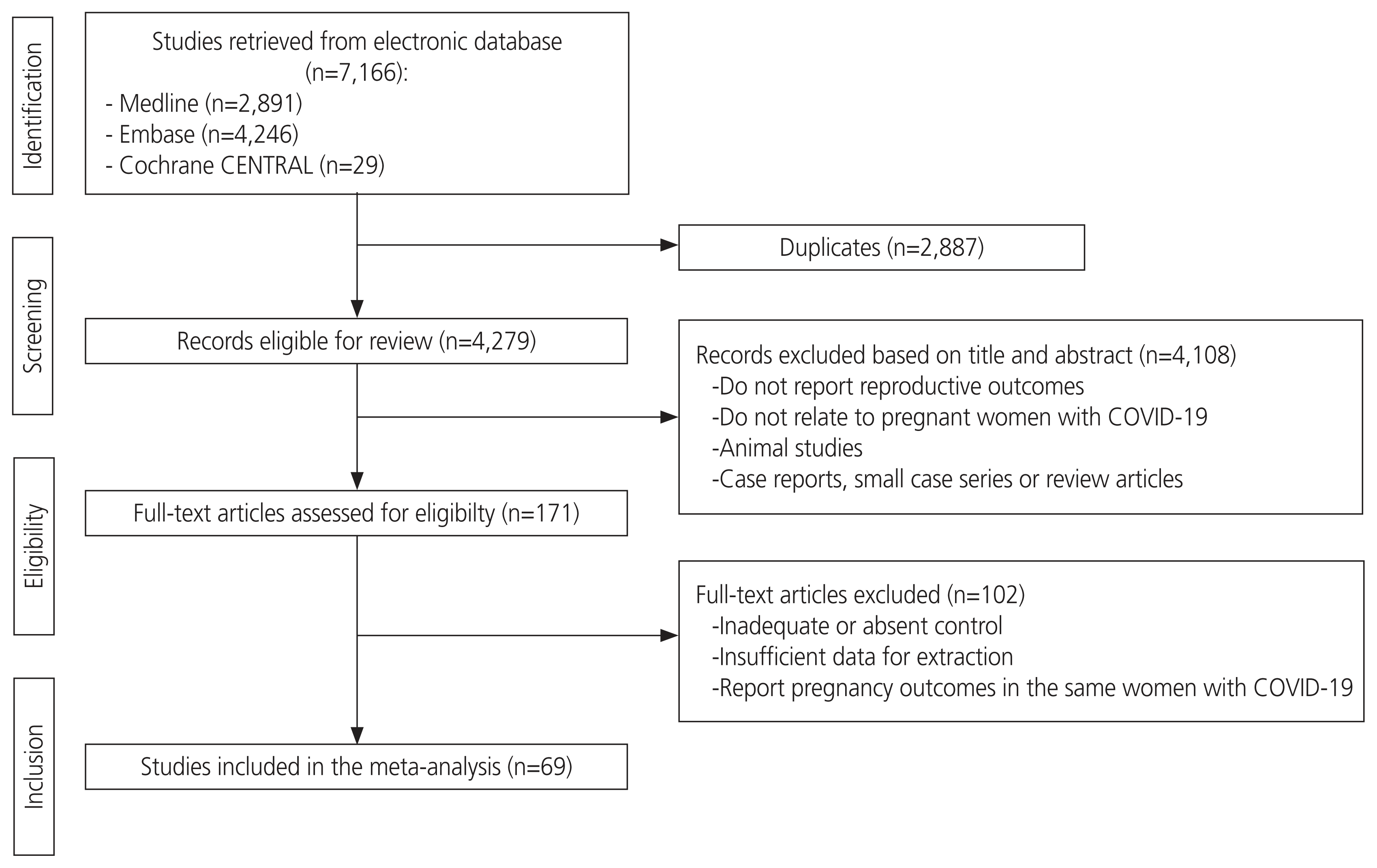
The database search identified 4,279 citations, of which 4,108 were excluded from the title and abstract review. The remaining 171 citations were considered eligible for full-text screening, of which 69 met the inclusion criteria and were considered eligible for inclusion in the meta-analysis (Fig. 1). Table 1 presents the baseline characteristics of the 69 included studies. These studies included 39,716 pregnant women with COVID-19 and 1,566,827 pregnant women without COVID-19. Reverse transcriptase-polymerase chain reaction was used as a diagnostic method to confirm COVID-19 in most studies [3,5–71].
Fifty studies reported the incidence of preterm births in 2,687 (10.3%) women with COVID-19 infection versus 58,203 (6.1%) women without the infection, demonstrating a significant difference (P<0.001). The risk of preterm birth was significantly (1.6-fold) higher among women with COVID-19 than those without (OR, 1.59; 95% CI, 1.42–1.78; I2=69%; Fig. 2A). Most studies did not accurately describe the indications for preterm birth. When only the eight studies that classified spontaneous preterm births were analyzed, the odds were 1.3-fold higher in women with COVID-19 than those without (OR, 1.33; 95% CI, 1.20–1.48; I2=22%; Fig. 2B).
Thirty-four studies reported that preeclampsia occurred in 2,214 of 27,732 women with COVID-19 (8.0%) versus 78,429 of 1,358,619 women without COVID-19 (5.8%), demonstrating a significant difference (OR, 1.41; 95% CI, 1.30–1.5; I2=31%; Fig. 3A).
Twelve studies reported that 987 neonates had LBW, including 407 born to women with COVID-19 and 580 born to women without COVID-19. The risk of LBW was significantly higher among the offspring of women with COVID-19 infection than those born to women without the infection (OR, 1.52; 95% CI, 1.30–1.79; I2=6%; Fig. 3B).
Fifty-seven studies reported the incidence of cesarean sections in 1,484,791 pregnant women. Women who experienced the COVID-19 infection during pregnancy were 1.2-fold more likely to deliver by a cesarean section than those without (OR, 1.20; 95% CI, 1.10–1.30; I2=79%; Fig. 4A).
Twenty-seven studies reported the incidence of IUFD or stillbirth in 256 women with COVID-19 and 6,730 women without COVID-19. The odds of IUFD or stillbirth were higher in women with COVID-19 than those without (OR, 1.71; 95% CI, 1.39–2.10; I2=27%; Fig. 4B).
Seven studies reported the incidence of fetal distress in 169 women with COVID-19 and 577 women without COVID-19. We observed a statistically significant increase in fetal distress among women with COVID-19 than those without (OR, 2.49; 95% CI, 1.54–4.03; I2=69%; Fig. 5A). Thirty-one studies reported the NICU admissions of 12,397 neonates (1,187 with COVID-19 and 11,210 without COVID-19). The overall risk of NICU admission was significantly higher among women with COVID-19 infection than those without (OR, 2.33; 95% CI, 1.72–3.16; I2=89%; Fig. 5B). Perinatal mortality occurred in 27 of 4,123 (0.65%) cases with COVID-19 versus 105 of 14,474 (0.73%) cases without COVID-19 among a total of 21 studies (OR, 1.96; 95% CI, 1.15–3.34; I2=0%; Fig. 5C).
Twelve studies reported the incidence of maternal mortality in 39 of 9,633 women (0.40%) with COVID-19 versus 90 of 494,811 women (0.02%) without COVID-19, revealing a significant difference between the two (P<0.05). COVID-19 infection during pregnancy resulted in an increased risk of maternal mortality relative to those who were not infected (OR, 6.15; 95% CI, 3.74; I2=62%; Fig. 5D).
Pregnant women with COVID-19 had a higher risk of placental abruption (OR, 1.40; 95% CI, 1.02–1.92; I2=19%; 12 studies), IUGR or SGA (OR, 1.12; 95% CI, 1.0–1.26; I2=48%; 27 studies), gestational diabetes mellitus (OR, 1.13; 95% CI, 1.04–1.23; I2=45%; 40 studies), and congenital anomalies (OR, 1.45; 95% CI, 1.04–2.01; I2=0%; eight studies) (Supplementary Fig. 1). No significant differences were observed in the total miscarriage rates (OR, 1.04; 95% CI, 0.64–1.60; I2=74%; 13 studies), the incidences of PPROM (OR, 1.36; 95% CI, 0.96–1.93; I2=26%; 10 studies), postpartum hemorrhage (OR, 0.98; 95% CI, 0.78–1.24; I2=70%; 22 studies), cholestasis (OR, 1.34; 95% CI, 0.83–2.18; I2=0%; seven studies), or chorioamnionitis (OR, 1.26; 95% CI, 0.93–1.72; I2=38%; 14 studies) between women with and without the COVID-19 infection (Supplementary Fig. 2).
COVID-19 has spread rapidly worldwide and become a global health concern. It is associated with various complications, including high mortality and morbidity rates ranging from pulmonary distress to death. Pregnant women are susceptible to respiratory infections and can experience adverse outcomes. In this updated systematic review and meta-analysis, we described the obstetric and neonatal outcomes of COVID-19-infected versus non-infected pregnant women. We also investigated the effects of COVID-19 infection during pregnancy on adverse pregnancy outcomes by comparing the obstetric and neonatal outcomes of COVID-19-infected versus non-infected pregnant women.
Adverse pregnancy outcomes were more common in pregnant women with COVID-19 than in those without. This review revealed that COVID-19 during pregnancy is associated with an increased risk of cesarean delivery, preterm birth, preeclampsia, IUFD or stillbirth, gestational diabetes mellitus, IUGR or SGA, and LBW. Moreover, COVID-19 infection during pregnancy can lead to an increased risk of neonatal complications, such as fetal distress, NICU admission, and perinatal mortality. Furthermore, COVID-19 negatively affected maternal mortality.
These findings are consistent with those of previous studies [72]. Allotey et al. [2] reported that COVID-19 infection during pregnancy increases the risk of preterm birth, LBW, NICU admission, and stillbirth. Lassi et al. [73] reported a 2-fold increased risk of preterm birth in women with severe symptomatic COVID-19. Pathirathna et al. [74] suggested that pregnant women with COVID-19 have an increased risk of maternal death, preeclampsia, cesarean delivery, fetal distress, preterm birth, LBW, NICU admission, and stillbirth. In contrast, Huntley et al. [75], and Wei et al. [76] reported that COVID-19 infection during pregnancy did not increase the risk of cesarean delivery. Nevertheless, Wei et al. [77] reported that the risk of preterm birth, cesarean delivery, and preeclampsia increased with the presence and severity of COVID-19 symptoms.
Previous studies have reported that the most common obstetric complications associated with COVID-19 are PPROM, postpartum hemorrhage, cesarean delivery, and preterm birth [78,79]. The inflammatory reaction associated with infection can lead to the destruction of placental function and the subsequent development of adverse pregnancy outcomes [18,58,80]. Cesarean section is the preferred delivery route in COVID-19-positive pregnancies. The higher rate of cesarean sections compared to the current cesarean delivery rate of 30% could be due to changes in the obstetric management protocols influenced by the infectious disease management policy of the institution; this could also be attributed to disease progression caused by COVID-19. This finding is consistent with the results of another meta-analysis. Since most studies examined did not specify the indications for cesarean sections, this could not be accurately analyzed.
The present review demonstrates that COVID-19 infection during pregnancy is associated with an increased risk of preeclampsia. Pérez-López et al. [81] reported that preeclampsia and hypertensive disorders of pregnancy were more common in COVID-19-infected pregnant women regardless of the presence or severity of their symptoms. Other studies reported a positive association between preeclampsia and COVID-19 during pregnancy [74,77,82,83]. Multiple mechanisms are involved in the pathogenesis of preeclampsia in pregnant women with COVID-19. SARS-CoV-2 binds to cell membrane angiotensin-converting enzyme 2, which plays an important role in the renin-angiotensin system (RAS). RAS controls trophoblast proliferation, angiogenesis, and uteroplacental blood flow. COVID-19 can alter RAS function via its downregulation, which induces preeclampsia. Moreover, the infected placenta shows a decreased expression of angiotensin-conversing enzyme 2 receptors concurrent with increased production of soluble fms-like tyrosine kinase-1, which has been implicated in the pathogenesis of preeclampsia. Garrido-Pontnou et al. [84], and Hecht et al. [85] reported that infected COVID-19 term placentas demonstrated villous trophoblast necrosis, inflammatory infiltration, and fibrinoid deposition. The authors suggest that these placental mechanisms against infection could contribute to adverse pregnancy outcomes.
Moreover, COVID-19 infection during pregnancy increases the risk of preterm birth. However, a previous study reported that the preterm birth rate during the pandemic was lower than that in the pre-pandemic era [86]. In this review, we did not address the causes of preterm birth, whether spontaneous or medically induced. Even when analyzing spontaneous preterm births, we observed that the risk of spontaneous preterm births was significantly higher in women with COVID-19 than in those without. Neonates born to women with COVID-19 had a higher NICU admission risk than those born to non-infected women. This finding is consistent with those of previous studies. Furthermore, COVID-19 increases the risk of fetal distress and perinatal mortality.
Pregnant women are categorized as immunosuppressed because they undergo immunological and physiological changes during pregnancy that prevent fetal allograft rejection [87]. Hence, pregnant women are more susceptible to respiratory infections than does the general population. Moreover, the physiological changes in the cardiopulmonary system in pregnant women can worsen the symptoms of respiratory infections. Furthermore, an elevated diaphragm, increased oxygen demand, and generalized edema, including that in the bronchial mucosa, experienced by pregnant women are risk factors for hypoxia [88,89]. Previously, influenza A subtype H1N1 virus, SARS-CoV, and MERS infections have demonstrated more systemic complications in pregnant women than in the general population [90,91]. However, unlike other respiratory viruses, the severity of COVID-19 remains relatively low. A previous study reported that the laboratory findings, clinical presentations, and radiological findings of pregnant women with COVID-19 were similar to those of non-pregnant women [11]. Several hypotheses have been proposed to explain these findings. One of them is that COVID-19 is caused by a novel ribonucleic acid mutation. Furthermore, concurrent with pregnancy, adaptive organ changes to offset fetal rejection may have resulted in a protective effect against the virus [92]. The process of immunological adaptation, which starts during pregnancy, may be the key to the milder symptoms of COVID-19 noted during pregnancy.
Vertical transmission was not considered in the present study. A previous review reported that vertical transmission of SARS-CoV-2 was rare and not detected in nasopharyngeal swabs of neonates, cord blood, or amniotic fluid [80]. Other studies have also reported very little evidence to support the vertical transmission of COVID-19 [81,83].
Although a few studies have investigated breastfeeding with COVID-19 infection, previous studies have reported conflicting results. Some researchers do not recommend breastfeeding when a nursing mother is infected, whereas others suggest that breastfeeding while wearing a mask should be practiced. Additionally, the virus is not believed to be transmitted through breast milk. However, further studies to confirm this hypothesis are warranted [80,93–96].
This review has several strengths. As the studies included in this review were extracted from multiple countries, our findings can be generalized globally and interpreted regardless of race or country. The present systematic review compared the pregnancy outcomes of COVID-19-infected and non-infected pregnant women and did not investigate the general population. The selection bias was low owing to the high threshold of the inclusion criteria. This review also included a larger number of studies than previous reports. This review demonstrated that COVID-19 has a worse effect on outcomes, such as preterm birth, cesarean section, and placental abruption, compared with the outcomes reported in previous studies.
This study also has several limitations. First, the disease remains prevalent globally, causing additional alterations in public health restrictions. However, prospective randomized studies on viral infections are not applicable to pregnant women. Therefore, we included only retrospective, nonrandomized studies in this review. Second, the studies included in this report were conducted in multiple countries, and their baseline characteristics were highly heterogeneous including the types of COVID-19 variants. Third, we were unable to access the raw data analyzed in these studies. Fourth, this study did not analyze infections according to pregnancy trimesters. The pregnancy trimester at the infection time could be a significant factor in adverse pregnancy outcomes, as respiratory infections in early pregnancy can critically impact organogenesis. Fifth, this review did not assess indications for the delivery route.
As the global spread of COVID-19 continues, it is imperative to determine further its impact on pregnancy outcomes to prepare for future outbreaks. Pregnant women are particularly susceptible to respiratory infections, which can lead to high pregnancy morbidity and mortality rates. The findings of this review and meta-analysis provide sufficient information to enable evidence-based counseling and help clinicians manage pregnant women with COVID-19.
Notes
References
1. Khoury R, Bernstein PS, Debolt C, Stone J, Sutton DM, Simpson LL, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstet Gynecol. 2020; 136:273–82.

2. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020; 370:m3320.

3. Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020; 3:e2029256.

4. Melo GC, Araújo KCGM. COVID-19 infection in pregnant women, preterm delivery, birth weight, and vertical transmission: a systematic review and meta-analysis. Cad Saude Publica. 2020; 36:e00087320.

5. Shephard HM, Manning SE, Nestoridi E, Brown C, Yazdy MM. Characteristics of people with and without laboratory-confirmed SARS-CoV-2 infection during pregnancy, massachusetts, March 2020–March 2021. Public Health Rep. 2022; 137:782–9.

6. Vera von Bargen H, Espinosa Serrano M, Martin Navarrete D, Ahumada Droguett P, Méndez Benavente C, Flores Castillo M, et al. Analysis of prevalence and sociodemographic conditions among women in labor with and without COVID-19 in public hospitals in Chile. J Perinat Med. 2021; 50:132–8.

7. Griffin I, Benarba F, Peters C, Oyelese Y, Murphy T, Contreras D, et al. The impact of COVID-19 infection on labor and delivery, newborn nursery, and neonatal intensive care unit: prospective observational data from a single hospital system. Am J Perinatol. 2020; 37:1022–30.

8. Wei L, Gao X, Chen S, Zeng W, Wu J, Lin X, et al. Clinical characteristics and outcomes of childbearing-age women with COVID-19 in Wuhan: retrospective, single-center study. J Med Internet Res. 2020; 22:e19642.

9. Martinez-Perez O, Prats Rodriguez P, Muner Hernandez M, Encinas Pardilla MB, Perez Perez N, Vila Hernandez MR, et al. The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021; 21:273.

10. Dhuyvetter A, Cejtin HE, Adam M, Patel A. Coronavirus disease 2019 in pregnancy: the experience at an urban safety net hospital. J Community Health. 2021; 46:267–9.

11. Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. 2020; 55:435–7.

12. Charles CM, Osman NB, Arijama D, Matingane B, Sitoé T, Kenga D, et al. Clinical and epidemiological aspects of SARS-CoV-2 infection among pregnant and postpartum women in Mozambique: a prospective cohort study. Reprod Health. 2022; 19:164.

13. Nayak AH, Kapote DS, Fonseca M, Chavan N, Mayekar R, Sarmalkar M, et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynaecol India. 2020; 70:256–61.

14. Taghavi SA, Heidari S, Jahanfar S, Amirjani S, Aji-ramkani A, Azizi-Kutenaee M, et al. Obstetric, maternal, and neonatal outcomes in COVID-19 compared to healthy pregnant women in Iran: a retrospective, case-control study. Middle East Fertil Soc J. 2021; 26:17.

15. Milln J, Heard S, Gunganah K, Velauthar L, Saeed F. Clinical characteristics and pregnancy outcomes of women diagnosed with SARS-CoV-2 in London’s most ethnically diverse borough: a cross-sectional study. Obstet Med. 2021; 14:164–9.

16. Pasternak B, Neovius M, Söderling J, Ahlberg M, Norman M, Ludvigsson JF, et al. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. 2021; 174:873–5.

17. Prabhu M, Cagino K, Matthews KC, Friedlander RL, Glynn SM, Kubiak JM, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020; 127:1548–56.

18. Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin Infect Dis. 2020; 71:2035–41.

19. Flaherman VJ, Afshar Y, Boscardin WJ, Keller RL, Mardy AH, Prahl MK, et al. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): first report from the pregnancy coronavirus outcomes registry (PRIORITY) study. Clin Infect Dis. 2021; 73:e2810–3.

20. Crovetto F, Crispi F, Llurba E, Pascal R, Larroya M, Trilla C, et al. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021; 73:1768–75.

21. Edlow AG, Li JZ, Collier AY, Atyeo C, James KE, Boatin AA, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020; 3:e2030455.

22. Rodríguez-Díaz M, Alonso-Molero J, Cabero-Perez MJ, Llorca J, Dierssen-Sotos T, Gómez-Acebo I, et al. Pregnancy and birth outcomes during the early months of the COVID-19 pandemic: the MOACC-19 cohort. Int J Environ Res Public Health. 2021; 18:10931.

23. Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021; 181:714–7.

24. Regan AK, Arah OA, Fell DB, Sullivan SG. SARS-CoV-2 infection during pregnancy and associated perinatal health outcomes: a national US cohort study. J Infect Dis. 2022; 225:759–67.

25. Sahin D, Tanacan A, Erol SA, Anuk AT, Eyi EGY, Ozgu-Erdinc AS, et al. A pandemic center’s experience of managing pregnant women with COVID-19 infection in Turkey: a prospective cohort study. Int J Gynaecol Obstet. 2020; 151:74–82.

26. Ferrugini CLP, Boldrini NAT, Costa FLS, Salgueiro MAOB, Coelho PDP, Miranda AE. SARS-CoV-2 infection in pregnant women assisted in a high-risk maternity hospital in Brazil: clinical aspects and obstetric outcomes. PLoS One. 2022; 17:e0264901.

27. Binte Masud S, Zebeen F, Alam DW, Hossian M, Zaman S, Begum RA, et al. Adverse birth outcomes among pregnant women with and without COVID-19: a comparative study from bangladesh. J Prev Med Public Health. 2021; 54:422–30.

28. Brandt JS, Fell DB. SARS-CoV-2 infection in pregnancy: lessons learned from the first pandemic wave. Paediatr Perinat Epidemiol. 2021; 35:34–6.

29. Hcini N, Maamri F, Picone O, Carod JF, Lambert V, Mathieu M, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021; 257:11–8.

30. Patberg ET, Adams T, Rekawek P, Vahanian SA, Akerman M, Hernandez A, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021; 224:382.e1–382.e18.

31. Sakowicz A, Ayala AE, Ukeje CC, Witting CS, Grobman WA, Miller ES. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM. 2020; 2:100198.

32. Pineles BL, Goodman KE, Pineles L, O’Hara LM, Nadimpalli G, Magder LS, et al. In-hospital mortality in a cohort of hospitalized pregnant and nonpregnant patients with COVID-19. Ann Intern Med. 2021; 174:1186–8.

33. Saadia Z, Farrukh R, Kanwal S, Shahzad Q. Clinical profiles, demographic features, and maternal outcomes among coronavirus disease positive pregnant women: a cross-sectional study. Open Access Maced J Med Sci. 2021; 9:486–91.

34. Díaz-Corvillón P, Mönckeberg M, Barros A, Illanes SE, Soldati A, Nien JK, et al. Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS One. 2020; 15:e0239887.

35. Fallach N, Segal Y, Agassy J, Perez G, Peretz A, Chodick G, et al. Pregnancy outcomes after SARS-CoV-2 infection by trimester: a large, population-based cohort study. PLoS One. 2022; 17:e0270893.

36. Sutton D, Wen T, Staniczenko AP, Huang Y, Andrikopoulou M, D’Alton M, et al. Clinical and demographic risk factors for COVID-19 during delivery hospitalizations in New York City. Am J Perinatol. 2021; 38:857–68.

37. Getahun D, Peltier MR, Lurvey LD, Shi JM, Braun D, Sacks DA, et al. Association between SARS-CoV-2 infection and adverse perinatal outcomes in a large health maintenance organization. Am J Perinatol. 2022; Jun. 23. [Epub]. https://doi.org/10.1055/s-0042-1749666.

38. Cruz-Lemini M, Ferriols Perez E, de la Cruz Conty ML, Caño Aguilar A, Encinas Pardilla MB, Prats Rodríguez P, et al. Obstetric outcomes of SARS-CoV-2 infection in asymptomatic pregnant women. Viruses. 2021; 13:112.

39. Cruz Melguizo S, de la Cruz Conty ML, Carmona Payán P, Abascal-Saiz A, Pintando Recarte P, González Rodríguez L, et al. Pregnancy outcomes and SARS-CoV-2 infection: the Spanish Obstetric Emergency Group study. Viruses. 2021; 13:853.

40. Teixeira MLB, Costa Ferreira Júnior OD, João E, Fuller T, Silva Esteves J, Mendes-Silva W, et al. Maternal and neonatal outcomes of SARS-CoV-2 infection in a cohort of pregnant women with comorbid disorders. Viruses. 2021; 13:1277.

41. Wang MJ, Schapero M, Iverson R, Yarrington CD. Obstetric hemorrhage risk associated with novel COVID-19 diagnosis from a single-institution cohort in the United States. Am J Perinatol. 2020; 37:1411–6.

42. Abedzadeh-Kalahroudi M, Karimian Z, Nasiri S, Khorshidifard MS. Anxiety and perceived stress of pregnant women towards covid-19 disease and its related factors in Kashan (2020). Iran J Obstet Gynecol Infertil. 2021; 24:8–18.
43. Alipour Z, Samadi P, Eskandari N, Ghaedrahmati M, Vahedian M, Khalajinia Z, et al. Relationship between coronavirus disease 2019 in pregnancy and maternal and fetal outcomes: retrospective analytical cohort study. Midwifery. 2021; 102:103128.

44. Li WK, Chen XJC, Altshuler D, Islam S, Spiegler P, Emerson L, et al. The incidence of propofol infusion syndrome in critically-ill patients. J Crit Care. 2022; 71:154098.

45. Cardona-Pérez JA, Villegas-Mota I, Helguera-Repetto AC, Acevedo-Gallegos S, Rodríguez-Bosch M, Aguinaga-Ríos M, et al. Prevalence, clinical features, and outcomes of SARS-CoV-2 infection in pregnant women with or without mild/moderate symptoms: results from universal screening in a tertiary care center in Mexico City, Mexico. PLoS One. 2021; 16:e0249584.

46. Chornock R, Iqbal SN, Wang T, Kodama S, Kawakita T, Fries M. Incidence of hypertensive disorders of pregnancy in women with COVID-19. Am J Perinatol. 2021; 38:766–72.

47. Cosma S, Carosso AR, Borella F, Cusato J, Bovetti M, Bevilacqua F, et al. Prenatal biochemical and ultrasound markers in covid-19 pregnant patients: a prospective case-control study. Diagnostics (Basel). 2021; 11:398.

48. Cuñarro-López Y, Cano-Valderrama Ó, Pintado-Recarte P, Cueto-Hernández I, González-Garzón B, García-Tizón S, et al. Maternal and perinatal outcomes in patients with suspected COVID-19 and their relationship with a negative RT-PCR result. J Clin Med. 2020; 9:3552.

49. Farghaly MAA, Kupferman F, Castillo F, Kim RM. Characteristics of newborns born to SARS-CoV-2-positive mothers: a retrospective cohort study. Am J Perinatol. 2020; 37:1310–6.

50. Gulersen M, Blitz MJ, Rochelson B, Nimaroff M, Shan W, Bornstein E. Clinical implications of SARS-CoV-2 infection in the viable preterm period. Am J Perinatol. 2020; 37:1077–83.

51. Gupta P, Khatana VP, Prabha R, Jha I, Singh M, Pandey AK, et al. An observational study for appraisal of clinical outcome and risk of mother-to-child SARS-CoV-2 transmission in neonates provided the benefits of mothers’ own milk. Eur J Pediatr. 2022; 181:513–27.

52. Gurol-Urganci I, Waite L, Webster K, Jardine J, Carroll F, Dunn G, et al. Obstetric interventions and pregnancy outcomes during the COVID-19 pandemic in England: a nationwide cohort study. PLoS Med. 2022; 19:e1003884.

53. Smithgall MC, Liu-Jarin X, Hamele-Bena D, Cimic A, Mourad M, Debelenko L, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020; 77:994–9.

54. Katz D, Bateman BT, Kjaer K, Turner DP, Spence NZ, Habib AS, et al. The society for obstetric anesthesia and perinatology coronavirus disease 2019 registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome coronavirus-2 outbreak in the United States. Anesth Analg. 2021; 133:462–73.

55. Ko JY, DeSisto CL, Simeone RM, Ellington S, Galang RR, Oduyebo T, et al. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis. 2021; 73:S24–31.

56. Laresgoiti-Servitje E, Cardona-Pérez JA, Hernández-Cruz RG, Helguera-Repetto AC, Valdespino-Vázquez MY, Moreno-Verduzco ER, et al. COVID-19 infection in pregnancy: PCR cycle thresholds, placental pathology, and perinatal outcomes. Viruses. 2021; 13:1884.

57. Liao X, Yang H, Kong J, Yang H. Chest CT findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Med J. 2020; 37:226–8.

58. Liu S, Dzakpasu S, Nelson C, Wei SQ, Little J, Scott H, et al. Pregnancy outcomes during the COVID-19 pandemic in Canada, March to August 2020. J Obstet Gynaecol Can. 2021; 43:1406–15.

59. Papageorghiou AT, Gunier RB, Villar J. The link between COVID-19 and preeclampsia. Am J Obstet Gynecol. 2022; 226:153–4.

60. Pirjani R, Rabiei M, Abiri A, Moini A. An overview on guidelines on COVID-19 virus and natural and assisted reproductive techniques pregnancies. Int J Fertil Steril. 2020; 14:264–71.
61. Ríos-Silva M, Murillo-Zamora E, Mendoza-Cano O, Trujillo X, Huerta M. COVID-19 mortality among pregnant women in Mexico: a retrospective cohort study. J Glob Health. 2020; 10:020512.

62. Rosenbloom JI, Raghuraman N, Carter EB, Kelly JC. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2021; 224:623–4.

63. Ruggiero M, Somigliana E, Tassis B, Li Piani L, Uceda Renteria S, Barbara G, et al. Clinical relevance of SARS-CoV-2 infection in late pregnancy. BMC Pregnancy Childbirth. 2021; 21:505.

64. Savirón-Cornudella R, Villalba A, Esteban LM, Tajada M, Rodríguez-Solanilla B, Andeyro-Garcia M, et al. Screening of severe acute respiratory syndrome coronavirus-2 infection during labor and delivery using polymerase chain reaction and immunoglobulin testing. Life Sci. 2021; 271:119200.

65. Soto-Torres E, Hernandez-Andrade E, Huntley E, Mendez-Figueroa H, Blackwell SC. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol. 2021; 58:111–20.
66. Steffen HA, Swartz SR, Jackson JB, Kenne KA, Ten Eyck PP, Merryman AS, et al. SARS-CoV-2 infection during pregnancy in a rural midwest all-delivery cohort and associated maternal and neonatal outcomes. Am J Perinatol. 2021; 38:614–21.

67. Timircan M, Bratosin F, Vidican I, Suciu O, Tirnea L, Avram V, et al. Exploring pregnancy outcomes associated with SARS-CoV-2 infection. Medicina (Kaunas). 2021; 57:796.

68. Trahan MJ, O’Farrell P, Mitric C, Desilets J, Bastrash MP, Malhame I, et al. 364 obstetrical and neonatal outcomes among pregnancies with SARS-CoV-2. Am J Obstet Gynecol. 2021; 224:S237.

69. Roffman J, Villarreal J, Pride R, Bazer O, Karmacharya R, Lerou P, et al. Assessing covid-19 effects on neurodevelopment (ACEND): preliminary findings in 12-month-old children exposed to maternal sars-cov-2 infection during pregnancy. Neuropsychopharmacology. 2021; 46:150–1.
70. Vousden N, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK obstetric surveillance system (UKOSS). PLoS One. 2021; 16:e0251123.

71. Kathuria P, Khetarpal A, Singh P, Khatana S, Yadav G, Ghuman NK. Role of birth companion in COVID-19: indispensable for her and an auxiliary hand for us. Pan Afr Med J. 2020; 37:62.

72. Ahn KH, Kim HI, Lee KS, Heo JS, Kim HY, Cho GJ, et al. COVID-19 and vaccination during pregnancy: a systematic analysis using Korea National Health Insurance claims data. Obstet Gynecol Sci. 2022; 65:487–501.

73. Lassi ZS, Ana A, Das JK, Salam RA, Padhani ZA, Irfan O, et al. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J Glob Health. 2021; 11:05018.

74. Pathirathna ML, Samarasekara BPP, Dasanayake TS, Saravanakumar P, Weerasekara I. Adverse perinatal outcomes in COVID-19 infected pregnant women: a systematic review and meta-analysis. Healthcare (Basel). 2022; 10:203.

75. Huntley BJF, Mulder IA, Di Mascio D, Vintzileos WS, Vintzileos AM, Berghella V, et al. Adverse pregnancy outcomes among individuals with and without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systematic review and meta-analysis. Obstet Gynecol. 2021; 137:585–96.
76. Wei WH, Mellis S, Geba GP, Sivapalasingam S, Jalbert JJ. Incidence and risk factors for COVID-related urgent medical visits among US patients diagnosed with COVID-19 in the outpatient setting. Pharmacoepidemiol Drug Saf. 2021; 30:22–3.
77. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021; 193:E540–8.

78. Bastos SNMAN, Barbosa BLF, Cruz LGB, Souza RP, Silva Melo SSE, Luz CCBDS. Clinical and obstetric aspects of pregnant women with COVID-19: a systematic review. Rev Bras Ginecol Obstet. 2021; 43:949–60.

79. Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol. 2021; 31:1–16.
80. Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, et al. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020; 49:418–23.
81. Pérez-López FR, Savirón-Cornudella R, Chedraui P, López-Baena MT, Pérez-Roncero G, Sanz-Arenal A, et al. Obstetric and perinatal outcomes of pregnancies with COVID 19: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022; 35:9742–58.
82. Wang H, Li N, Sun C, Guo X, Su W, Song Q, et al. The association between pregnancy and COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2022; 56:188–95.
83. Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022; 226:68–89.e3.
84. Garrido-Pontnou M, Navarro A, Camacho J, Crispi F, Alguacil-Guillén M, Moreno-Baró A, et al. Diffuse trophoblast damage is the hallmark of SARS-CoV-2-associated fetal demise. Mod Pathol. 2021; 34:1704–9.
85. Hecht JL, Quade B, Deshpande V, Mino-Kenudson M, Ting DT, Desai N, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020; 33:2092–103.

86. Berghella V, Boelig R, Roman A, Burd J, Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020; 2:100258.

88. Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021; 101:303–18.

90. Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004; 191:292–7.

91. Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle east respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019; 52:501–3.

92. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006; 12:1638–43.

93. Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei province. Zhonghua Fu Chan Ke Za Zhi. 2020; 55:166–71.
94. Fan C, Lei D, Fang C, Li C, Wang M, Liu Y, et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin Infect Dis. 2021; 72:862–4.

Fig. 2
Forest plots of the summary crude odds ratios and 95% confidence intervals for the association between coronavirus disease 2019 and preterm delivery (A) and spontaneous preterm delivery (B). SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; CI, confidence interval.
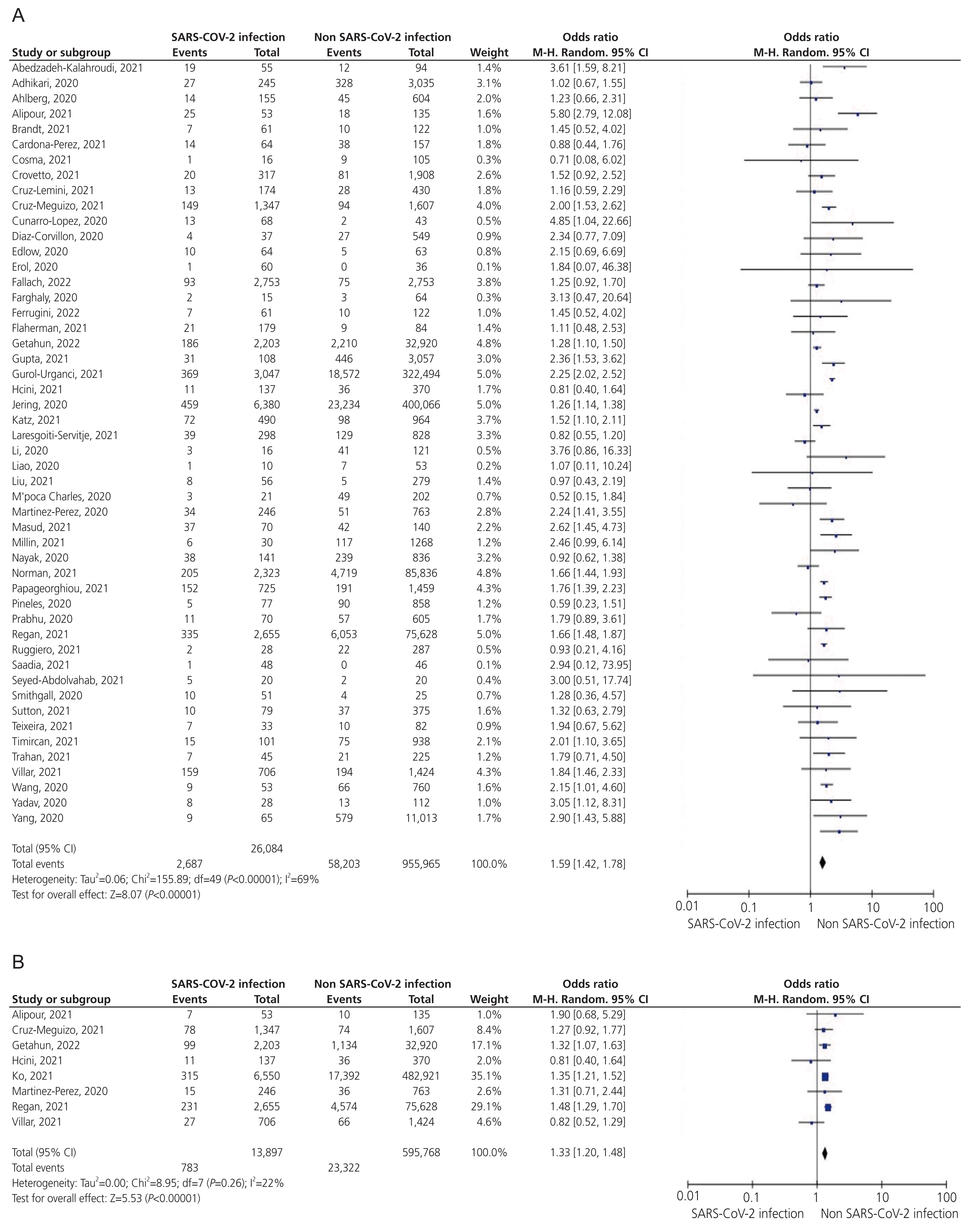
Fig. 3
Forest plots of the summary crude odds ratios and 95% confidence intervals for the association between coronavirus disease 2019 and preeclampsia (A) and low birth weight (B). SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; CI, confidence interval.
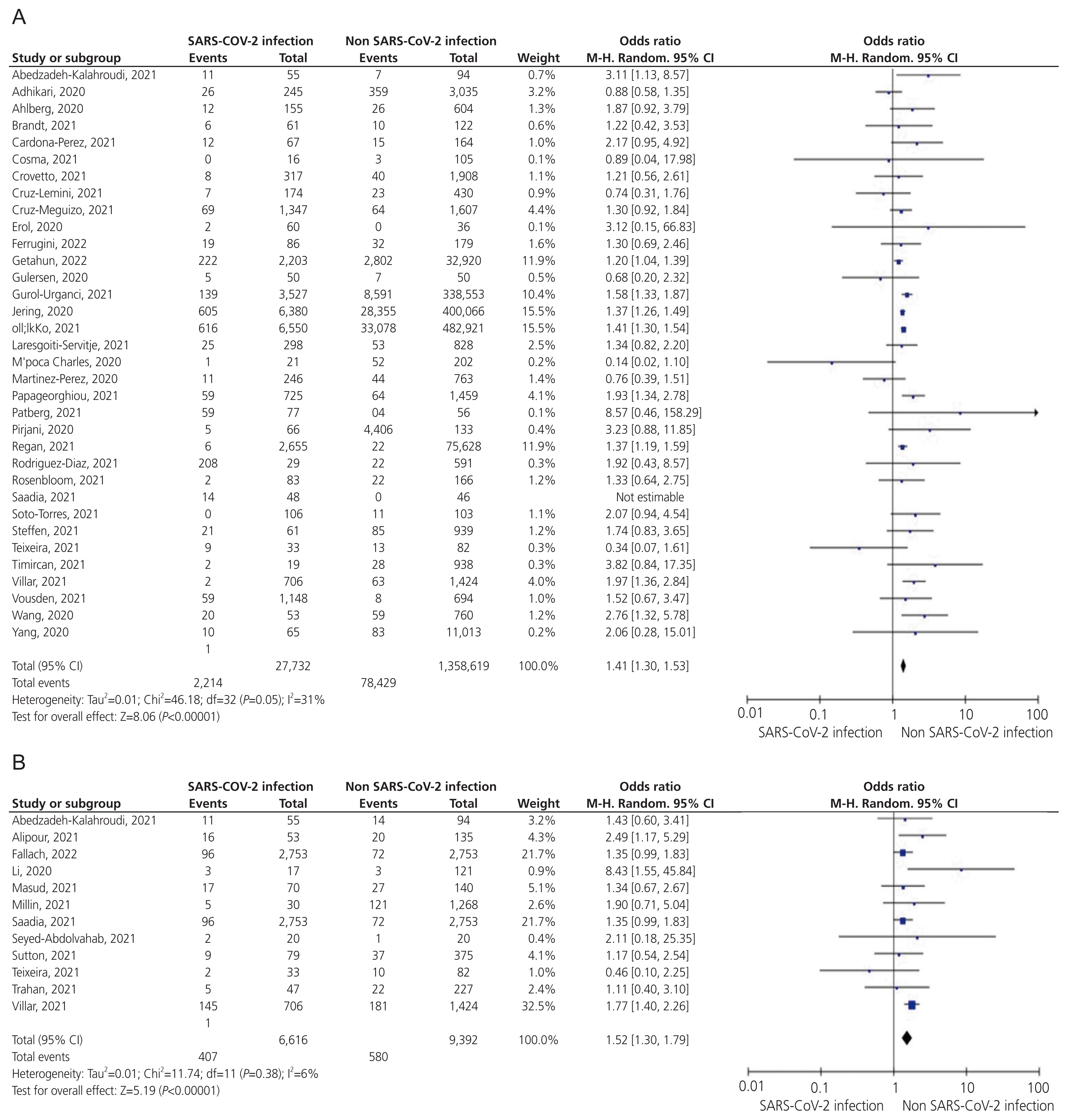
Fig. 4
Forest plots of the summary crude odds ratios and 95% confidence intervals for the association between coronavirus disease 2019 and cesarean section (A) and IUFD or stillbirth (B). SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; CI, confidence interval; IUFD, intrauterine fetal death.
Fig. 5
Forest plots of the summary crude odds ratios and 95% confidence intervals for the association between coronavirus disease 2019 and fetal distress (A), NICU admission (B), perinatal mortality (C), and maternal mortality (D). SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; CI, confidence interval; NICU, neonatal intensive care unit.
Table 1
Baseline characteristics of included studies




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download