Abstract
Purpose
Reports showed that some of intrahepatic cholangiocarcinoma (ICC) patients with lymph node metastasis (LNM) may also gain survival benefit undergone resection. However, the effect of the extent of LNM on prognosis and surgical indication is barely discussed.
Methods
From September 1994 to November 2018, primary ICC patients undergone initial curable surgery were enrolled. Based on the extent of LNM, we divided these patients into 4 groups, including patients with no LNM (group N0), LNM to hepatoduodenal ligament or common hepatic artery (region A, group A), LNM to gastrohepatic lymph nodes for left liver ICC and periduodenal and peripancreatic lymph node for right liver ICC (region B, group B), or LNM beyond these regions (region C, group C). Multivariable Cox regression analysis was performed to identify the prognostic factors for recurrence-free survival (RFS) and overall survival (OS) in all groups.
Results
A total of 133 patients were enrolled. There were 56, 21, 17, and 39 patients in groups N0, A, B, and C, respectively. There was significant difference between groups N0 and C in RFS (P < 0.001) and OS (P = 0.002). When we compared group N0 + A + B with group C, we also found that RFS (P < 0.001) and OS (P = 0.007) were significantly different. In multivariable analysis, the extent of LNM was an independent risk factor for RFS (P < 0.050).
Intrahepatic cholangiocarcinoma (ICC) is a highly aggressive malignant tumor that originates from the endothelial cells above the second-order bile ducts [1]. It accounts for 10%–20% of primary liver cancers and the incidence is rising [23]. Most ICC patients have advanced-stage disease at diagnosis, and the 5-year overall survival (OS) rates are lower than 5%–10% [3].
ICC is prone to have lymph node metastasis (LNM). About 25%–40% of ICC patients undergone simultaneous lymph node (LN) resection have been pathologically confirmed LNM [4]. It has been reported that LNM is an independent risk factor for prognosis. In a multicenter study, it was confirmed that LNM was a strong risk factor [5]. Patients’ prognosis cannot be stratified by tumor number and vascular invasion when they had LNM. In another meta-analysis [6], LNM was a dominant factor predicting shorter OS based on relevant literatures.
Surgical resection remains the mainstay of potentially curative therapy with median disease-free survival time of 12–36 months [78]. According to the latest National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Hepatobiliary Cancer [9], distant (beyond the porta hepatic) LNM contraindicates surgery as it generally indicates advanced incurable disease. However, it was reported that some ICC patients whose LNM beyond porta hepatic [10] and even to paraaortic [11] could still survive for a long time after radical surgery. So, the relationship between the extent of LNM and prognosis is not clear.
Because the lymphatic outflow system of liver and biliary tract is multidirectional and complex, there has been no study directly investigating on the pathway of LNM in ICC. Currently, most studies put forward the presumption of LN reflux pathways by calculating the number of positive LNs in different locations. The NCCN guidelines defined porta hepatic LNs as regional LNs, but relevant supporting literature is limited. In the periportal lymphatic system, lymphatic vessels run in Glisson’s sheath along with the portal vein. This periportal hepatic lymph flows in the same direction as bile, and 80% or more of hepatic lymph drains through this periportal lymphatic system [1213]. The efferent lymphatic vessels outside the liver communicate with hilar LNs and peripancreatic LNs and act as the first LN station. Based on the data of 39 ICC, Tsuji et al. [14] found that ICC mainly spread to the LNs in the hepatoduodenal ligament, then to those in the retropancreatic area, or around common hepatic artery. Okami et al. [15] found that for the ICC in the left hepatic lobe, the LNs along the lesser gastric curvature should also be included in the regional LNs. Studies above suggested that for ICC in the right lobe, the LNs around pancreas, for ICC in the left lobe, the LNs in the lesser curvature of the stomach should be included in the regional LNs.
Few researches have studied the prognosis of ICC with LNM beyond the porta hepatic after radical surgery. In this article, the influence of LNM in different regions according to the NCCN guidelines on the prognosis of ICC patients undergoing radical resection were analyzed.
Data on patients who underwent partial hepatectomy for ICC between September 1994 and November 2018 at 2 centers (The First Affiliated Hospital of Sun Yat-sen University and Sun Yat-sen University Cancer Center) were retrospectively analyzed.
Inclusion criteria included the following: (1) initial ICC patients who received primary radical surgery (R0/1); (2) at least porta hepatic LN dissection during operation; (3) histopathologically proven ICC; and (4) complete and available clinicopathologic data.
Exclusion criteria were as follows: (1) palliative surgery; (2) perioperative mortality; (3) hilar or extrahepatic cholangiocarcinoma, combined hepatocellular cholangiocarcinoma; (4) recurrent ICC, Tis, Tx; (5) received prior treatment before surgery; and (6) no dissection of LN during operation, including LN sampling.
The Institutional Ethical Review Committees of Clinical Research in the 2 centers have approved this study (No. [2022]501). Written informed consent was waived due to the retrospective design. The interventions were conducted in accordance with the Declaration of Helsinki and current ethical guidelines.
A detailed history, complete physical and hematological examination were performed to all patients. Other routine investigations included chest X-ray, abdominal ultrasound and/or contrast-enhanced ultrasound, contrast-enhanced CT and/or enhanced MRI, and if distant metastasis is suspected clinically or radiologically, PET was performed. A preoperative diagnosis of ICC was based on clinical symptoms, hematological markers, and imaging examinations.
Partial hepatectomy was performed after comprehensive assessment of tumor size, location, presence of cirrhosis, and estimated volume of liver remnant for each patient. Partial hepatectomy includes segments, sectors, and hemilivers. Direct invasion of adjacent structures and local extrahepatic metastasis and newly discovered intrahepatic lesions intraoperatively were removed whenever possible. Hepaticojejunostomy was carried out in patients with tumor involving the primary and secondary bile ducts.
Routine dissection of LNs in the porta hepatic, including hepatoduodenal ligament and common hepatic artery, was performed during operation. The extent of dissection was performed based on preoperative imaging and intraoperative exploration [161718]. LNs dissected during surgery would be accurately labeled with their corresponding anatomical locations. Eventually, the status of LNM was identified through pathologic examination, so as to determine the extent of LNM.
Histopathologic study of the resected specimens was carried out independently by 2 pathologists who came to a consensus by discussion if there was any controversy. Pathologic features, such as tumor diameter, number, type, capsule, location, surgical margin, number and locations of harvested LN, vascular invasion, and liver cirrhosis, were recorded.
In our study, the extent of postoperative LNM confirmed by pathology was divided into the following 3 regions. Region A included hepatoduodenal ligament and common hepatic artery LNs. Region B included gastrohepatic LNs for left liver ICC and periduodenal and peripancreatic LN for right liver ICC. Region C included nodes beyond these regions above, including the celiac, periaortic, and/or pericaval LNs. Patients with no LNM, with LNM to region A, region B, and region C were defined as group N0, group A, group B, and group C, respectively (Supplementary Fig. 1).
Patients were observed once every month in the first 6 months after surgery and every 3 months in the next 6 months, and then every 6 months thereafter. At each follow-up visit, a detailed history and complete physical examination were carried out. Blood routine, liver function, and tumor marker were tested. An abdominal ultrasound was also carried out. Contrast-enhanced CT or MRI was performed once every 6 months or earlier when tumor recurrence or metastasis was suspected. ICC recurrence/metastasis was defined as the appearance of a newly detected tumor confirmed on 2 radiologic images, with or without elevation of serum tumor markers. OS and recurrence-free survival (RFS) were used as primary end points. OS was defined as the interval between surgery and death or the last date of follow-up. RFS was calculated from surgery to the date when recurrence/metastasis was diagnosed.
Summary statistics were obtained using established methods and presented as percentages, mean, or median values. Cumulative event rates were calculated using the Kaplan-Meier method. Univariable and multivariable Cox proportional hazards models were developed using relevant clinicopathologic variables to determine the association of each with OS. Relative risks were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Significance levels were set at P < 0.05; all tests were 2-sided. All statistical analyses were performed using PASW Statistics ver. 17.0 (IBM Corp.).
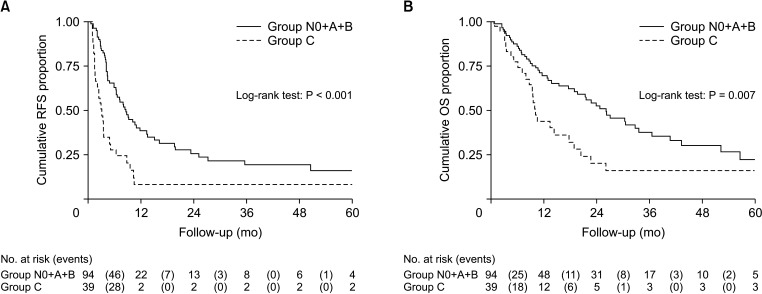
In this study, 321 patients with ICC who received partial hepatectomy were reviewed. One hundred seventy-six patients who did not undergo LN dissection (including only LN sampling) and 12 patients missing follow-up data were excluded. Finally, 133 patients were enrolled in our study. There were 56, 21, 17, and 39 patients in groups N0, A, B, and C, respectively. A total of 26 patients (19.5%) suffered postoperative complications, and the most common complication was fever (46.2%). A number of 10 patients (7.5%) received postoperative adjuvant therapy, 80.0% of which were chemotherapy. Complications and adjuvant treatment did not present statistical differences among the groups (Table 1).
The clinicopathologic characteristics are listed in Table 1. Compared with group N0, group N1 (group A + B + C) had higher level of CEA (P = 0.050) and CA 125 (P = 0.026), and higher number of LNs harvested (P = 0.003) (Supplementary Table 1). Groups A and B had higher number of LNs harvested compared to group N0 (P = 0.010 and P = 0.024) (Supplementary Tables 2, 3). Interestingly, group C had higher CA 125 (P = 0.045), larger tumor size (P = 0.049), and higher number of LNs harvested (P = 0.030) when compared with group N0 (Supplementary Table 4). Furthermore, group C had larger tumor size (P = 0.030) and a higher proportion of vascular invasion (P = 0.046) than group N0 + A + B (Supplementary Table 5). There was statistically different in the number of LNs harvested among group N0, A + B, and C (P = 0.006) (Supplementary Table 6).
After a median follow-up of 32.86 months (95% CI, 26.38–44.96 months), 70 patients recurred, and 77 patients died. For the 133 patients, the median RFS was 6.41 months (95% CI, 4.43–8.83 months), with 1-, 2-, and 3-year rates of 39.6%, 29.1%, and 22.8%. The median OS was 20.53 months (95% CI, 13.52–26.21 months), with 1-, 2-, and 3-year rates of 62.6%, 43.6%, and 32.9%, respectively.
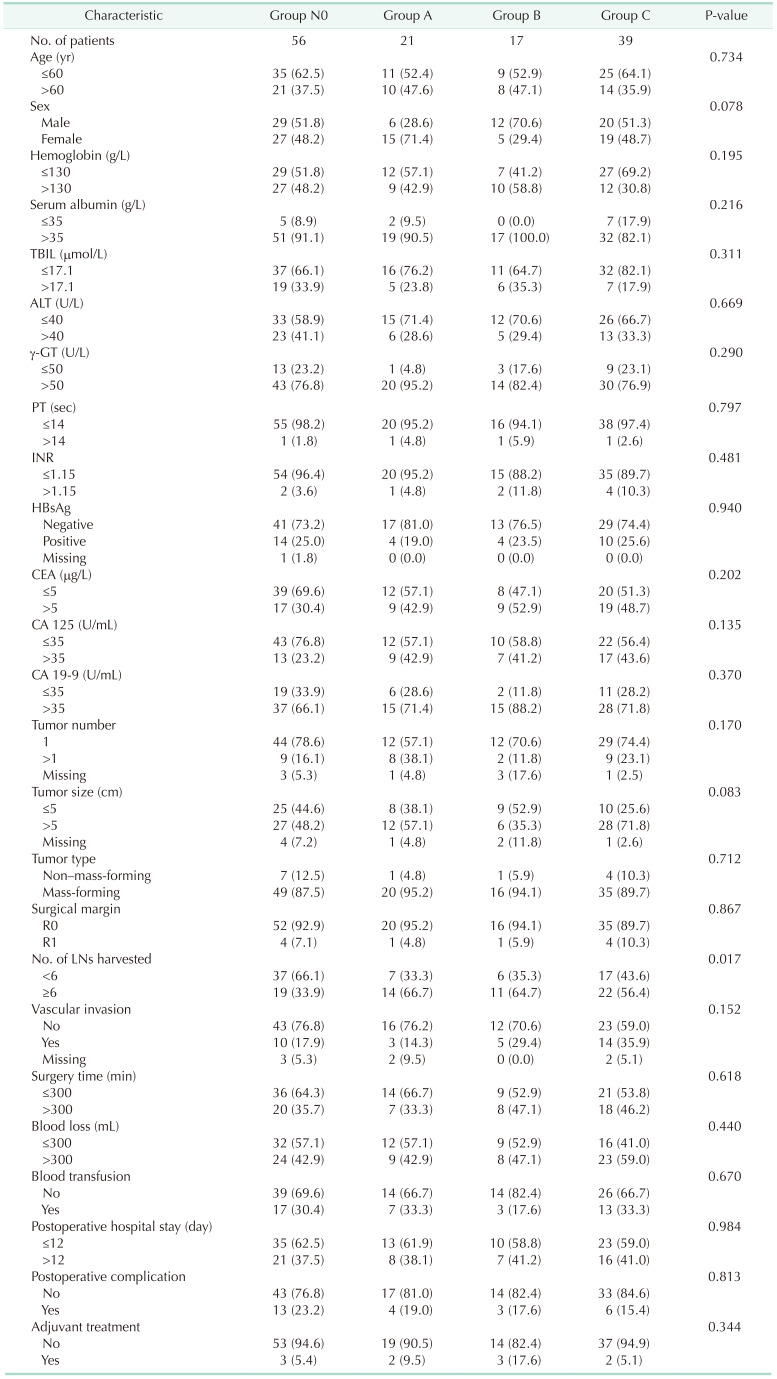
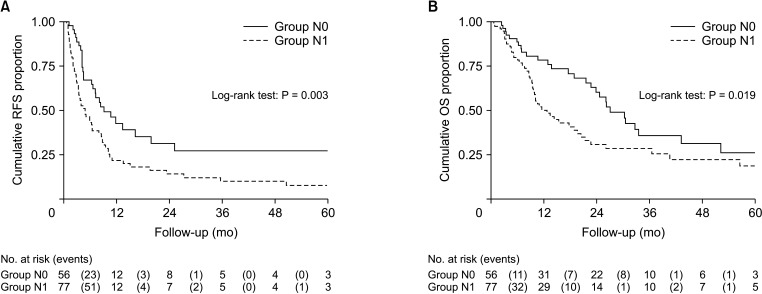
The median RFS and OS were 4.89 months (95% CI, 3.40–7.97 months) and 12.79 months (95% CI, 9.55–19.70 months) for group N1, and 9.12 months (95% CI, 6.38–19.83 months) and 27.07 months (95% CI, 21.65–43.30 months) for group N0. Significant difference was found between groups N0 and N1 in RFS (P = 0.003) and OS (P = 0.019) (Fig. 1). However, there was no significant difference in RFS and OS between groups A and N0 (P = 0.104 and P = 0.347) (Supplementary Fig. 2), groups B and N0 (P = 0.431 and P = 0.404) (Supplementary Fig. 3). The median RFS and OS of group C were 3.21 months (95% CI, 1.72–4.89 months) and 10.15 months (95% CI, 8.00–17.79 months), respectively. Significant difference could be observed between groups C and N0 in RFS (P < 0.001) and OS (P = 0.002) (Fig. 2). There was significant difference among groups N0, A + B, and C in RFS (P < 0.001) and OS (P = 0.014) (Supplementary Fig. 4). Nevertheless, no statistical differences in RFS (P = 0.134) and OS (P = 0.267) were detected between groups N0 and A + B (Supplementary Fig. 5).
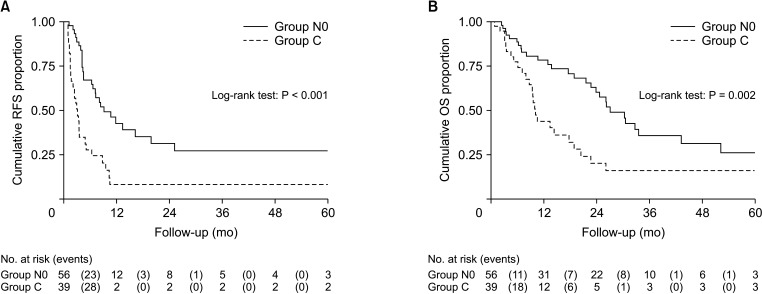
Based on the results above, we combined groups N0, A, and B to a larger group, and a comparative study was then conducted between the newly-established group (group N0 + A + B) and group C. The median RFS of group N0 + A + B was 8.60 months (95% CI, 6.38–11.87 months). Additionally, the median OS was 26.18 months (95% CI, 18.98–33.62 months). There was significant difference between the 2 groups in RFS (P < 0.001) and OS (P = 0.007) (Fig. 3).
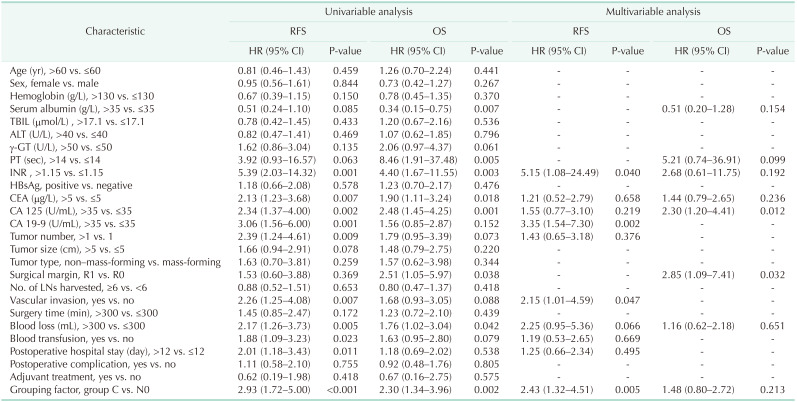
When we compared groups C and N0 (Table 2), the multivariable analysis indicated that the grouping factor, which indicated the extent of LNM, was an independent risk factor of RFS (HR, 2.43; 95% CI, 1.32–4.51; P = 0.005). Furthermore, international normalized ratio (INR; HR, 5.15; 95% CI, 1.08–24.49; P = 0.040), CA 19-9 (HR, 3.35; 95% CI, 1.54–7.30; P = 0.002), and vascular invasion (HR, 2.15; 95% CI, 1.01–4.59; P = 0.047) were also negative factors of RFS. The grouping factor was not an independent risk factor (P = 0.213) of OS in multivariable analysis although it was a negative factor (HR, 2.30; 95% CI, 1.34–3.96; P = 0.002) in univariable analysis. The factors that actually had influence on OS were CA 125 (HR, 2.30; 95% CI, 1.20–4.41; P = 0.012) and surgical margin (HR, 2.85; 95% CI, 1.09–7.41; P = 0.032).
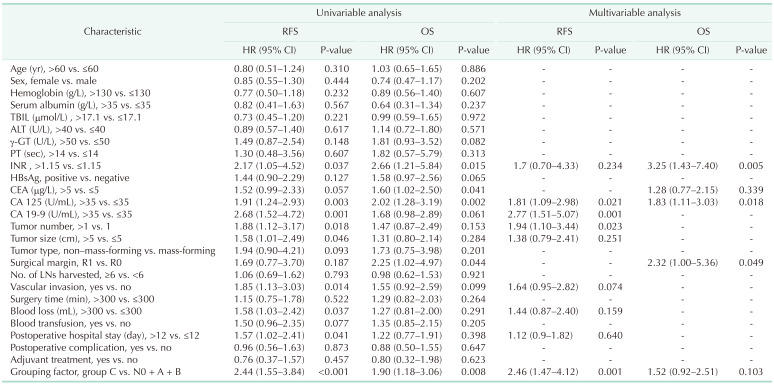
When we compared group N0 + A + B with group C (Table 3), the multivariable analysis revealed that the grouping factor (HR, 2.46; 95% CI, 1.47–4.12; P = 0.001), CA 125 (HR, 1.81; 95% CI, 1.09–2.98; P = 0.021), CA 19-9 (HR, 2.77; 95% CI, 1.51–5.07; P = 0.001), and tumor number (HR, 1.94; 95% CI, 1.10–3.44; P = 0.023) were significant risk factors for poor RFS. Univariable analysis showed that the grouping factor was a negative factor for OS (HR, 1.90; 95% CI, 1.18–3.06; P = 0.008), but in multivariable analysis, grouping factor was not an independent risk factors for OS (P = 0.103). INR (HR, 3.25; 95% CI, 1.43–7.40; P = 0.005), CA 125 (HR, 1.83; 95% CI, 1.11–3.03; P = 0.018), and surgical margin (HR, 2.32; 95% CI, 1.00–5.36; P = 0.049) were independent risk factors for OS.
Finally, when we compared groups C, A + B, and N0 (Supplementary Table 7), the multivariable analysis demonstrated that the group C alone was an independent risk factor of RFS (HR, 2.10; 95% CI, 1.21–3.65; P = 0.008). Meanwhile, CA 125 (HR, 1.73; 95% CI, 1.08–2.79; P = 0.023), CA 19-9 (HR, 3.00; 95% CI, 1.66–5.44; P < 0.001), and tumor number (HR, 2.13; 95% CI, 1.22–3.70; P = 0.007) were also negative factors of RFS. However, the grouping factor was not an independent risk factor (all P > 0.05) of OS in multivariable analysis, although group C was a negative factor (HR, 2.19; 95% CI, 1.28–3.74; P = 0.004) in univariable analysis. The factors that had an impact on OS were INR (HR, 3.21; 95% CI, 1.37–7.54; P = 0.007), CA 125 (HR, 1.82; 95% CI, 1.09–3.05; P = 0.022), and surgical margin (HR, 2.32; 95% CI, 1.01–5.36; P = 0.049).
Many studies have demonstrated that LNM is an independent risk factor for the prognosis of ICC. To our knowledge, although some studies have investigated on lymph outflow pathway of liver and ICC and evaluated different aspects of LNM such as the total harvested nodes [19], the number of involved nodes [20], the ratio of positive to total LNs and log odds of metastatic nodes [21]. However, few researches focused on assessing the effect of the extent of LNM on prognosis of ICC and evaluated the prognosis of ICC with LNM beyond the porta hepatic after surgery. In this article, according to the NCCN guidelines and relevant studies, we divided LNs into different regions to evaluate the effect of the extent of LNM on the prognosis of ICC patients undergoing radical resection.
The median survival time was reported to be 11.0–37.4 months [2223242526], and these values were similar to those of our study. In our study, we found that ICC patients with LNM beyond the regional LNs defined by NCCN guidelines, including gastrohepatic, periduodenal and peripancreatic LN, could still achieve satisfactory prognosis comparable to patients without LNM after surgery. However, when LN metastasized beyond the extent mentioned above, patients had a significantly shorter RFS and OS.
It has been previously reported that ICC patients has a much poorer prognosis once they have LNM, regardless of the extent of LN metastasis. The main reasons are as follows. Firstly, LNM always spreads to distant LNs and is seldom limited to the regional LNs [27]. Secondly, it is believed that LNM is a systemic disease, and LN dissection alone is not likely to improve the prognosis without further control of recurrence [28]. However, in our study, 38 patients (49.4%) in group N1 had LNM limited to region A and region B, and the prognosis of these patients after radical surgery was comparable to that of patients in group N0, with satisfying RFS and OS, which indicated that not all patients in group N1 have a poor prognosis. We could stratify patients in group N1 according to the extent of LNM.
NCCN guidelines define regional LNs as porta hepatic LNs, and LNM beyond this region is considered as distant LNs. Surgical resection is not recommended when patients with ICC have distant LN metastasis. But this recommendation of NCCN guidelines lacks strong support. The results of our study suggested that according to the prognostic information, gastrohepatic, periduodenal, and peripancreatic LNs could still be classified as regional LNs, which is consistent with the lymphatic outflow pathway of liver and ICC. Lymphatic vessels outside the liver communicate with hilar LNs and peripancreatic LNs and act as the first LN station and the left type of ICC tends to spread along the left gastric nodes through the lesser curvature of the stomach, which suggests that the ICC in the right lobe of the liver, the peripancreatic LNs [1113], and the LNs around the lesser curvature of the stomach of the ICC in the left lobe of the liver, should be included in the regional LNs [1415].
There are several limitations of this study. Firstly, this is a retrospective study, and the sample size is relatively small, which may bring bias to the results. Secondly, about a quarter of the patients (n = 32, 24.1%) had positive hepatitis B surface antigen in our study while HCV infection and primary sclerosing cholangitis are important factors in carcinogenesis of ICC, especially in Western countries. Thus, whether this result is applicable to patients with a Western background is still unclear. Thirdly, in this study, the assessment of the extent of LN dissection was based on the imaging and surgical exploration. Although enhanced CT or PET can give a high negative predictive value (nearly 99%) in patients without lymphadenopathy and our criteria have been adopted by other studies [717], this is still a limitation that might affect the results to a certain extent.
In conclusion, ICC patients with LNM beyond the regional LNs defined by NCCN, including gastrohepatic, periduodenal and peripancreatic LN, could still achieve good prognosis after radical surgery. However, when LNM beyond the range mentioned above, surgery should be carefully considered.
Notes
Fund/Grant Support: This work was supported by grants from the National Natural Science Foundation of China for Youths (No. 81900546), Science and Technology Program of Guangzhou, China (No. 201704020215), and Chen-Xiaoping foundation for the development of science and technology of Hubei province-fund for Hepatology-pancreatobiliary tumor (No. CXPJJH11900001-2019322).
References
1. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma: evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018; 15:95–111. PMID: 28994423.
2. Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, et al. Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology. 2019; 69:1803–1815. PMID: 30251463.
3. Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004; 24:115–125. PMID: 15192785.
4. Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg. 2018; 22:52–59. PMID: 28424987.
5. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29:3140–3145. PMID: 21730269.
6. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014; 149:565–574. PMID: 24718873.
7. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013; 31:1188–1195. PMID: 23358969.
8. Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009; 16:3048–3056. PMID: 19626372.
9. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: hepatobiliary cancers, version 2.2022 [Internet]. NCCN;2022. updated 2022 Jul 15. cited 2022 Aug 31. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
.
10. Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021; 28:26–54. PMID: 33259690.
11. Trutmann M, Sasse D. The lymphatics of the liver. Anat Embryol (Berl). 1994; 190:201–209. PMID: 7818092.
12. Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015; 50:913–927. PMID: 25833009.
13. Pupulim LF, Vilgrain V, Ronot M, Becker CD, Breguet R, Terraz S. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging. 2015; 40:1997–2011. PMID: 25579171.
14. Tsuji T, Hiraoka T, Kanemitsu K, Takamori H, Tanabe D, Tashiro S. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001; 129:401–407. PMID: 11283529.
15. Okami J, Dono K, Sakon M, Tsujie M, Hayashi N, Fujiwara Y, et al. Patterns of regional lymph node involvement in intrahepatic cholangiocarcinoma of the left lobe. J Gastrointest Surg. 2003; 7:850–856. PMID: 14592657.
16. Noji T, Kondo S, Hirano S, Tanaka E, Ambo Y, Kawarada Y, et al. CT evaluation of paraaortic lymph node metastasis in patients with biliary cancer. J Gastroenterol. 2005; 40:739–743. PMID: 16082591.
17. Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D’Angelica M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006; 244:260–264. PMID: 16858189.
18. Noji T, Kondo S, Hirano S, Tanaka E, Suzuki O, Shichinohe T. Computed tomography evaluation of regional lymph node metastases in patients with biliary cancer. Br J Surg. 2008; 95:92–96. PMID: 17853509.
19. Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010; 252:107–114. PMID: 20531002.
20. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005; 29:728–733. PMID: 15880276.
21. Kim Y, Spolverato G, Amini N, Margonis GA, Gupta R, Ejaz A, et al. Surgical management of intrahepatic cholangiocarcinoma: defining an optimal prognostic lymph node stratification schema. Ann Surg Oncol. 2015; 22:2772–2778. PMID: 25663595.
22. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016; 18:79–87. PMID: 26776855.
23. Guglielmi A, Ruzzenente A, Campagnaro T, Valdegamberi A, Bagante F, Bertuzzo F, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013; 17:1917–1928. PMID: 24048613.
24. Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002; 89:1525–1531. PMID: 12445060.
25. Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001; 193:384–391. PMID: 11584966.
26. Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004; 91:99–104. PMID: 14716802.
27. Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001; 88:1463–1466. PMID: 11683741.
28. Yamamoto M, Takasaki K, Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999; 29:147–150. PMID: 10225697.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–7 and Supplementary Figs. 1–5 can be found via https://doi.org/10.4174/astr.2023.104.5.258.
Supplementary Table 1
Demographics and clinicopathologic characteristics of patients between groups N0 and N1 (A + B + C)
Supplementary Table 2
Demographics and clinicopathologic characteristics of patients between groups N0 and A
Supplementary Table 3
Demographics and clinicopathologic characteristics of patients between groups N0 and B
Supplementary Table 4
Demographics and clinicopathologic characteristics of patients between groups N0 and C
Supplementary Table 5
Demographics and clinicopathologic characteristics of patients between groups N0 + A + B and C
Supplementary Table 6
Demographics and clinicopathologic characteristics of patients among groups N0, A + B, and C
Supplementary Table 7
Univariable and multivariable analysis of prognosis factors for RFS and OS among groups N0, A + B, and C
Supplementary Fig. 1
Schematic figure of grouping of extent of lymph node metastasis (LNM). LNM appearing in the brown lymph nodes region is group A, appearing in the yellow lymph nodes region is group B, and exceeding these regions belongs to group C.
Supplementary Fig. 2
Kaplan-Meier survival curves of patients in groups N0 (n = 56) and A (n = 21). (A) Recurrence-free survival (RFS) for patients in groups N0 and A (P = 0.104). (B) Overall survival (OS) for patients in groups N0 and A (P = 0.347).
Supplementary Fig. 3
Kaplan-Meier survival curves of patients in groups N0 (n = 56) and B (n = 17). (A) Recurrence-free survival (RFS) for patients in groups N0 and B (P = 0.431). (B) Overall survival (OS) for patients in groups N0 and B (P = 0.404).
Supplementary Fig. 4
Kaplan-Meier survival curves of patients in groups N0 (n = 56), A + B (n = 38), and C (n = 39). (A) Recurrence-free survival (RFS) for patients in groups N0, A + B, and C (P < 0.001). (B) Overall survival (OS) for patients in groups N0, A + B, and C (P = 0.014).
Supplementary Fig. 5
Kaplan-Meier survival curves of patients in groups N0 (n = 56) and A + B (n = 38). (A) Recurrence-free survival (RFS) for patients in groups N0 and A + B (P = 0.134). (B) Overall survival (OS) for patients in groups N0 and A + B (P = 0.267).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download