1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020; 91(1):157–160. PMID:
32191675.
2. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020; 55(3):105924. PMID:
32081636.

3. Thakur V, Kanta Ratho R. Omicron (B. 1.1. 529): a new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. 2022; 94(5):1821–1824. PMID:
34936120.

4. Rahimi F, Talebi Bezmin Abadi A. Omicron: a highly transmissible SARS-CoV-2 variant. Gene Rep. 2022; 27:101549. PMID:
35165664.

5. Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022; 386(7):698–700. PMID:
35021005.

6. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the Omicron (B. 1.1. 529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID:
35249272.

7. Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022; 28(3):477–480. PMID:
35046572.

8. Zhou R, To KK, Peng Q, Chan JM, Huang H, Yang D, et al. Vaccine-breakthrough infection by the SARS-CoV-2 Omicron variant elicits broadly cross-reactive immune responses. Clin Transl Med. 2022; 12(1):e720. PMID:
35083867.

9. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022; 327(6):583–584. PMID:
34967859.

10. Christensen PA, Olsen RJ, Long SW, Snehal R, Davis JJ, Ojeda Saavedra M, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022; 192(4):642–652. PMID:
35123975.

11. Shin DH, Oh HS, Jang H, Lee S, Choi BS, Kim D. Analyses of confirmed COVID-19 cases among Korean military personnel after mass vaccination. J Korean Med Sci. 2022; 37(3):e23. PMID:
35040298.

12. Choe PG, Kim Y, Chang E, Kang CK, Kim NJ, Cho NH, et al. Kinetics of neutralizing antibody responses against SARS-CoV-2 Delta Variant in patients infected at the beginning of the pandemic. J Korean Med Sci. 2022; 37(8):e67. PMID:
35226425.

13. Yi S, Kim JM, Choe YJ, Hong S, Choi S, Ahn SB, et al. SARS-CoV-2 Delta variant breakthrough infection and onward secondary transmission in household. J Korean Med Sci. 2022; 37(1):e12. PMID:
34981682.

14. Um J, Choi YY, Kim G, Kim MK, Lee KS, Sung HK, et al. Booster BNT162b2 COVID-19 vaccination increases neutralizing antibody titers against the SARS-CoV-2 Omicron variant in both young and elderly adults. J Korean Med Sci. 2022; 37(9):e70. PMID:
35257525.

15. Kwon SR, Kim N, Park H, Minn D, Park S, Roh EY, et al. Strong SARS-CoV-2 antibody response after booster dose of BNT162b2 mRNA vaccines in uninfected healthcare workers. J Korean Med Sci. 2022; 37(19):e135. PMID:
35578582.

16. Mohapatra RK, Tiwari R, Sarangi AK, Islam MR, Chakraborty C, Dhama K. Omicron (B.1.1.529) variant of SARS-CoV-2: concerns, challenges, and recent updates. J Med Virol. 2022; 94(6):2336–2342. PMID:
35118666.

17. Mahase E.. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021; 375:n2713. PMID:
34750163.

18. Korea Disease Control and Prevention Agency. Regular Briefing (February 14, 2022). Cheongju: Korea Disease Control and Prevention Agency;2022.
19. Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B. 1.1. 529 (Omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021; 36(50):e346. PMID:
34962117.

22. Roosa K, Lee Y, Luo R, Kirpich A, Rothenberg R, Hyman JM, et al. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect Dis Model. 2020; 5:256–263. PMID:
32110742.

23. Kim S, Seo YB, Jung E. Prediction of COVID-19 transmission dynamics using a mathematical model considering behavior changes in Korea. Epidemiol Health. 2020; 42:e2020026. PMID:
32375455.
24. Tuite AR, Fisman DN, Greer AL. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. CMAJ. 2020; 192(19):E497–E505. PMID:
32269018.

25. Sarkar K, Khajanchi S, Nieto JJ. Modeling and forecasting the COVID-19 pandemic in India. Chaos Solitons Fractals. 2020; 139:110049. PMID:
32834603.

26. Kucharski AJ, Klepac P, Conlan AJ, Kissler SM, Tang ML, Fry H, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020; 20(10):1151–1160. PMID:
32559451.

27. Kretzschmar ME, Rozhnova G, Bootsma MC, van Boven M, van de Wijgert JH, Bonten MJ. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020; 5(8):e452–e459. PMID:
32682487.

28. Kim S, Ko Y, Kim YJ, Jung E. The impact of social distancing and public behavior changes on COVID-19 transmission dynamics in the Republic of Korea. PLoS One. 2020; 15(9):e0238684. PMID:
32970716.

29. Kim S, Kim YJ, Peck KR, Jung E. School opening delay effect on transmission dynamics of coronavirus disease 2019 in Korea: based on mathematical modeling and simulation study. J Korean Med Sci. 2020; 35(13):e143. PMID:
32242349.

30. Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, et al. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020; 5:293–308. PMID:
32355904.

31. Bubar KM, Reinholt K, Kissler SM, Lipsitch M, Cobey S, Grad YH, et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021; 371(6532):916–921. PMID:
33479118.

32. Ko Y, Lee J, Kim Y, Kwon D, Jung E. COVID-19 vaccine priority strategy using a heterogenous transmission model based on maximum likelihood estimation in the Republic of Korea. Int J Environ Res Public Health. 2021; 18(12):6469. PMID:
34203821.

33. Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021; 21(6):793–802. PMID:
33743847.

34. Min KD, Tak S. Dynamics of the COVID-19 epidemic in the post-vaccination period in Korea: a rapid assessment. Epidemiol Health. 2021; 43:e2021040. PMID:
34082498.

35. Murayama H, Kayano T, Nishiura H. Estimating COVID-19 cases infected with the variant alpha (VOC 202012/01): an analysis of screening data in Tokyo, January-March 2021. Theor Biol Med Model. 2021; 18(1):13. PMID:
34273991.

36. Sonabend R, Whittles LK, Imai N, Perez-Guzman PN, Knock ES, Rawson T, et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: a mathematical modelling study. Lancet. 2021; 398(10313):1825–1835. PMID:
34717829.

38. Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. COVID-19 vaccine effectiveness in New York state. N Engl J Med. 2022; 386(2):116–127. PMID:
34942067.

39. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021; 27(7):1205–1211. PMID:
34002089.

40. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022; 386(13):1207–1220. PMID:
35172051.

41. Korea Disease Control and Prevention Agency. Regular Briefing (February 3, 2022). Cheongju: Korea Disease Control and Prevention Agency;2022.
42. Jo Y, Kim SB, Radnaabaatar M, Huh K, Yoo JH, Peck KR, et al. Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea. Epidemiol Health. 2022; e2022034. PMID:
35381167.

43. Korea Disease Control and Prevention Agency. Regular Briefing (March 14, 2022). Cheongju: Korea Disease Control and Prevention Agency;2022.
45. Seoul Metropolitan Government. The Daily News Review of COVID-19 (March 4, 2022). Seoul: Seoul Metropolitan Government;2022.




 PDF
PDF Citation
Citation Print
Print



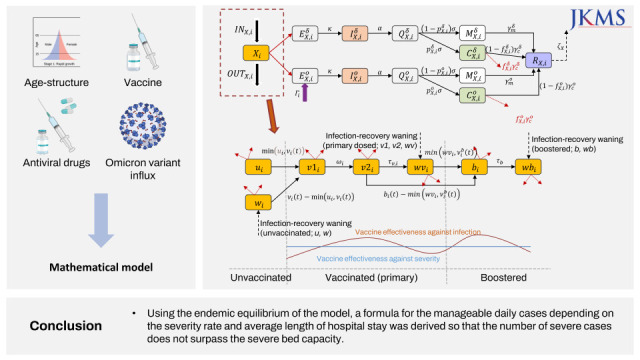
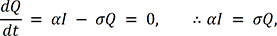
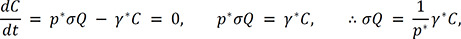
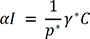
 .
.
 XML Download
XML Download