Abstract
Background
Methods
Results
Notes
Funding
CM received financial assistance from the Dr. Thomas Coonan Anesthesia, Pain & Perioperative Medicine Studentship and JJ Carroll Travel Fund Award through the Dalhousie Medical Research Foundation (Halifax, Canada).
Author Contributions
Jonathan G. Bailey (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft)
Catherine W Morgan (Data curation; Formal analysis; Funding acquisition; Project administration; Writing – review & editing)
Russell Christie (Data curation; Formal analysis; Investigation; Writing – review & editing)
Janny Xue Chen Ke (Data curation; Formal analysis; Investigation; Writing – review & editing)
M. Kwesi Kwofie (Conceptualization; Writing – review & editing)
Vishal Uppal (Conceptualization; Formal analysis; Methodology; Writing – review & editing)
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
References
Fig. 1.
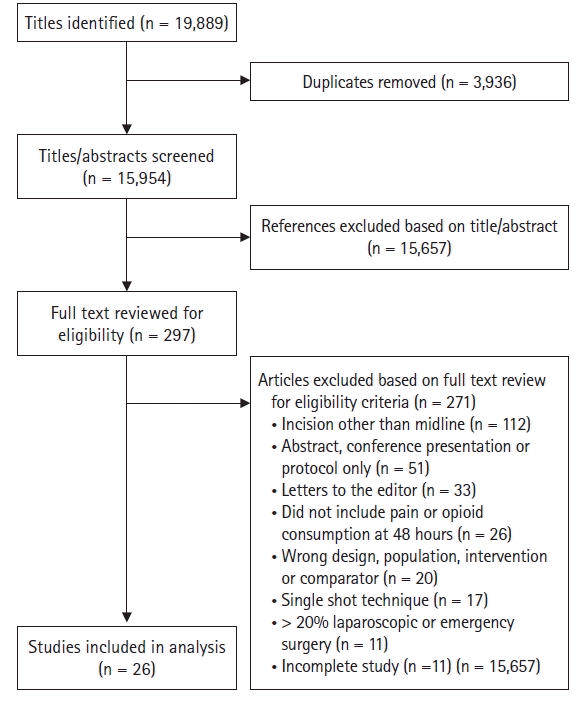
Fig. 2.
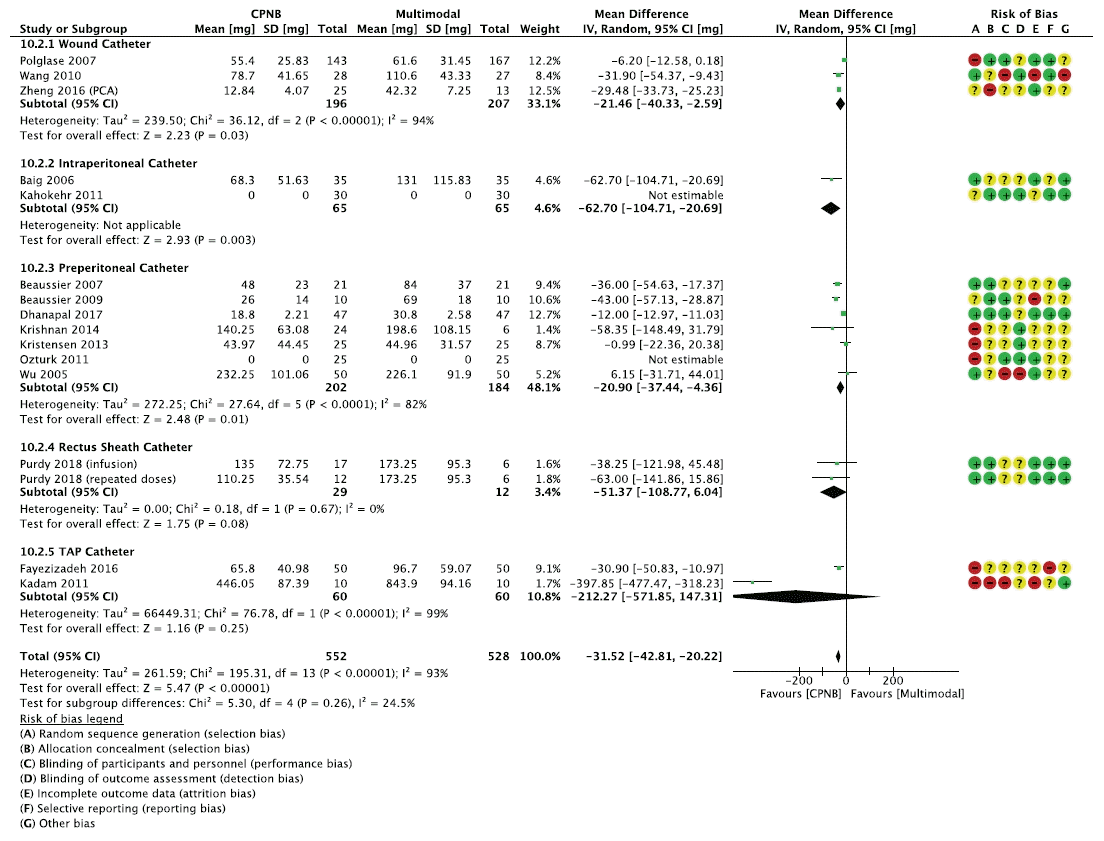
Fig. 3.
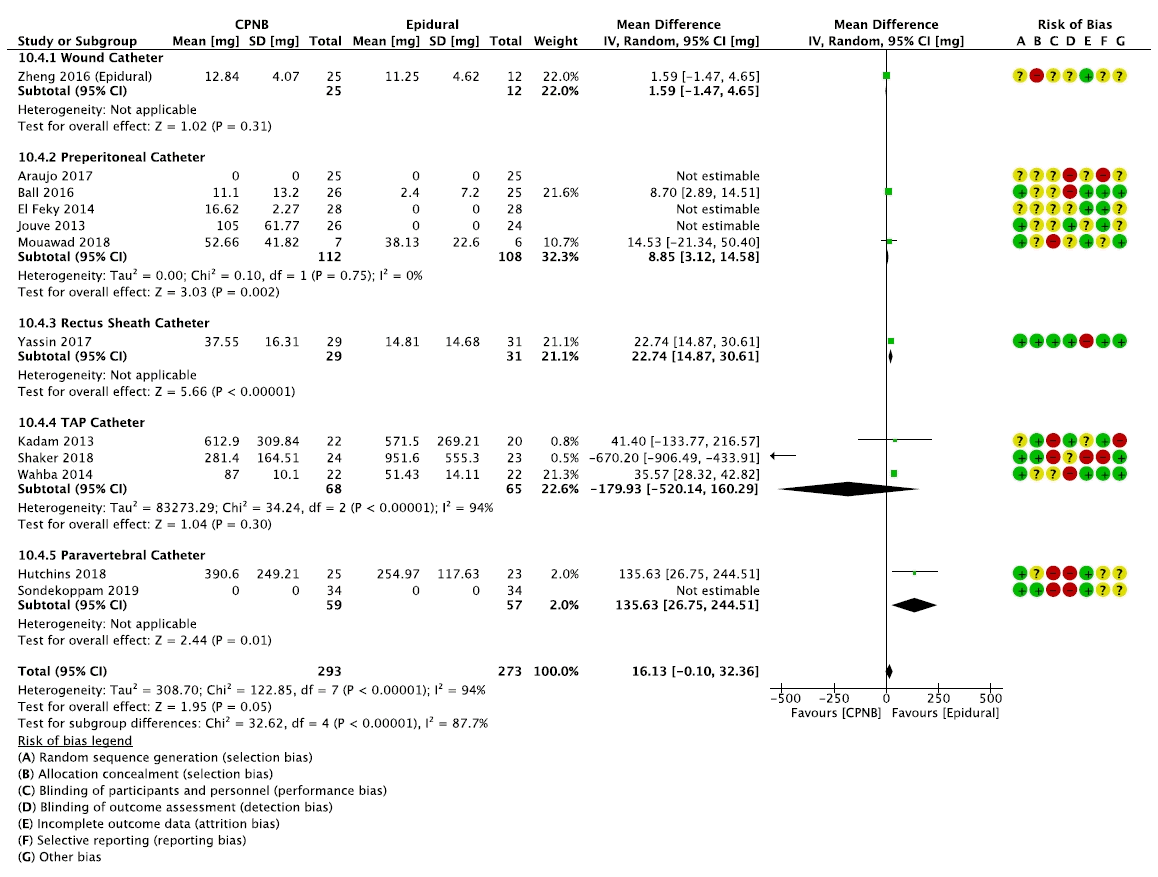
Table 1.
|
n |
Age |
Sex |
BMI |
Local anesthetic |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | CPNB | Control |
Mean (SD) years |
% (male) |
Mean (SD) m2/kg |
Agent | Infusion (ml/h) | Intermittent (ml, qh) | Surgical procedure | |||||||||
| CPNB | Control | CPNB | Control | CPNB type | Placed by | ||||||||||||||
| A. Multimodal | |||||||||||||||||||
| Polglase 2007 | Australia | 143 | 167 | 67 | 14 | 65 | 13 | 48 | 53 | NR | NR | NR | NR | Wound | Surgeon | Ropi 0.5% | 4 | n/a | Colorectal |
| Wang 2010 | Australia | 28 | 27 | 65 | 23 | 70 | 22 | 57 | 52 | NR | NR | NR | NR | Wound | Surgeon | Ropi 0.2% | 4 | n/a | Colorectal |
| Zheng 2016* | China | 25 | 13 | 62 | 13 | 64 | 10 | 64 | 68 | 23 | 2 | 23 | 4 | Wound | Surgeon | Ropi 0.3% | 5 | n/a | Gastrectomy |
| Baig 2006 | USA | 35 | 35 | 56 | 18 | 59 | 16 | 57 | 77 | 25 | 4 | 27 | 5 | Intraperitoneal | Surgeon | Ropi 0.5% | 4 | n/a | Colectomy |
| Kahokehr 2011 | New Zealand | 30 | 30 | 63 | 15 | 63 | 19 | 33 | 43 | 29 | 5 | 27 | 5 | Intraperitoneal | NR | Ropi 0.2% | 8 | n/a | Colectomy |
| Beaussier 2007 | France | 21 | 21 | 58 | 10 | 62 | 9 | 67 | 52 | NR | NR | NR | NR | Preperitoneal | Surgeon | Ropi 0.2% | 10 | n/a | Colorectal |
| Beaussier 2009 | France | 10 | 10 | 57 | 15 | 55 | 15 | 50 | 50 | NR | NR | NR | NR | Preperitoneal | Surgeon | Ropi 0.2% | 10 | n/a | Colorectal |
| Dhanapal 2017 | India | 47 | 47 | 53 | 13 | 54 | 11 | 66 | 68 | 21 | 1 | 21 | 1 | Preperitoneal | Surgeon | Bupi 0.25% | 6 | n/a | UGI, Hemicolectomy |
| Krishnan 2014 | Australia | 24 | 6 | 66 | 11 | 65 | 19 | 58 | 67 | 29 | 6 | 28 | 6 | Preperitoneal | Surgeon | Levobupi 0.25% | 10 | n/a | Colorectal |
| Kristensen 2013 | Denmark | 25 | 25 | 64 | 4 | 66 | 5 | 100 | 100 | NR | NR | NR | NR | Preperitoneal | Surgeon | Bupi 0.25% | 5 | n/a | Prostatectomy |
| Ozturk 2011 | Turkey | 25 | 25 | 59 | 12 | 56 | 12 | 36 | 48 | 27 | 4 | 26 | 4 | Preperitoneal | Surgeon | Levobupi 0.25% | n/a | 10 ml q12h | Colorectal |
| Wu 2005 | USA | 50 | 50 | 55 | 7 | 57 | 7 | 100 | 100 | NR | NR | NR | NR | Preperitoneal | Surgeon | Bupi 0.5% | 2 | n/a | Prostatectomy |
| Purdy 2018† | Finland | 17 | 6 | 58 | 12 | 61 | 14 | 12 | 33 | 25 | 4 | 28 | 3 | Rectus | Surgeon | Levobupi 0.125% | 10 | n/a | Multiple |
| Purdy 2018‡ | Finland | 12 | 6 | 62 | 10 | 61 | 14 | 25 | 33 | 25 | 4 | 28 | 3 | Rectus | Surgeon | Levobupi 0.125% | n/a | 25 mg q4h | Multiple |
| Fayezizadeh 2016 | USA | 50 | 50 | 60 | 12 | 59 | 14 | 64 | 28 | 36 | 8 | 36 | 8 | TAP | Surgeon | LB + Bupi 0.25% | n/a | n/a | Ventral hernia |
| Kadam 2011 | Australia | 10 | 10 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | TAP | Anesthesia | Ropi 0.2% | 8–10 | n/a | Multiple |
| B. Epidural | |||||||||||||||||||
| Zheng 2016§ | China | 25 | 12 | 62 | 13 | 62 | 10 | 64 | 56 | 23 | 2 | 23 | 3 | Wound | Surgeon | Ropi 0.3% | 5 | n/a | Gastrectomy |
| Araujo 2017 | Portugal | 25 | 25 | 68 | 12 | 62 | 18 | 36 | 44 | NR | NR | NR | NR | Preperitoneal | Surgeon | Ropi 0.2% | 10 | n/a | Colorectal, |
| UGI, HPB | |||||||||||||||||||
| Ball 2016 | Italy | 26 | 25 | 73 | 3 | 74 | 2 | 92 | 84 | 26 | 3 | 27 | 3 | Preperitoneal | Surgeon | Levobupi 0.25% | 4 | n/a | Open AAA |
| El Feky 2014 | Egypt | 28 | 28 | 59 | 4 | 59 | 4 | 64 | 57 | NR | NR | NR | NR | Preperitoneal | Surgeon | Bupi 0.25% | 10 | n/a | Colorectal |
| Jouve 2013 | France | 26 | 24 | 68 | 9 | 63 | 12 | 50 | 54 | NR | NR | NR | NR | Preperitoneal | Surgeon | Ropi 0.2% | 10 | n/a | Colorectal |
| Mouawad 2018 | USA | 7 | 6 | 56 | 17 | 60 | 16 | 43 | 67 | 31 | 10 | 32 | 8 | Preperitoneal | Surgeon | Bupi 0.5% | 4–5 | n/a | Colorectal |
| Rao Kadam 2013 | Australia | 22 | 20 | 64 | 10 | 61 | 13 | 64 | 70 | NR | NR | NR | NR | TAP | Anesthesia | Ropi 0.2% | 8 | n/a | Colorectal, urology, UGI |
| Shaker 2018 | USA | 24 | 23 | 59 | 13 | 62 | 14 | NR | NR | 29 | 8 | 28 | 4 | TAP | Anesthesia | LB + Bupi 0.5% | n/a | n/a | Multiple |
| Wahba 2014 | Egypt | 22 | 22 | 66 | 5 | 66 | 5 | 50 | 45 | 31 | 4 | 31 | 3 | TAP | Anesthesia | Bupi 0.25% | n/a | 30 ml q8h | Colorectal, UGI, hernia |
| Yassin 2017 | Egypt | 29 | 31 | 48 | 9 | 48 | 12 | 23 | 21 | 28 | 2 | 28 | 1 | Rectus | Anesthesia | Bupi 0.25% | n/a | 20 ml q8hr | UGI, HPB |
| Hutchins 2018 | USA | 25 | 23 | 68 | 12 | 64 | 9 | 48 | 61 | NR | NR | NR | NR | Paravertebral | Anesthesia | Ropi 0.2% | 14 | n/a | Pancreatectomy |
| Sondekoppam 2019 | Canada | 34 | 34 | 60 | 14 | 62 | 13 | 65 | 56 | 28 | 6 | 27 | 5 | Paravertebral | Anesthesia | Ropi 0.2% | 7 | n/a | Colorectal, HPB |
AAA: aortic abdominal aneurysm, BMI: body mass index, Bupi: bupivacaine, CPNB: continuous peripheral nerve blockade, HPB: hepatobiliary, LB: liposomal bupivacaine, Levobupi: levobupivacaine, n: number, N/a: not applicable, NR: not reported, Ropi: ropivacaine, SD: standard deviation, TAP: transversus abdominus plane, UGI: upper gastrointestinal.
Table 2.
| Outcomes | Anticipated absolute effects∗ (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care | Risk with CPNB | |||||
| Pain assessed with: Numeric Rating Scale | The mean pain score was 2.1 | MD 0.35 lower (0.77 lower to 0.07 higher) | - | 909 (9 RCTs, 1 cohort) | ⨁⨁◯◯ | The evidence suggests that CPNB results in little to no difference in pain. |
| Low†,‡ | ||||||
| Opioid consumption assessed with: Oral morphine equivalents | The mean opioid consumption was 92.9 mg | MD 31.52 mg lower (42.81 lower to 20.22 lower) | - | 970 (11 RCTs, 2 cohorts) | ⨁⨁◯◯ | The evidence suggests CPNB reduces opioid consumption. |
| Low†,‡ | ||||||
| Length of hospital stay assessed with: days | The mean length of hospital stay was 7.2 d | MD 1.41 d lower (2.45 lower to 0.36 lower) | - | 770 (7 RCTs, 1 cohort) | ⨁⨁◯◯ | The evidence suggests CPNB reduces length of hospital stay. |
| Low†,‡ | ||||||
| Postoperative nausea and vomiting | 127 per 1,000 | 113 per 1,000 (91 to 140) | RR 0.89 (0.72 to 1.10) | 568 (7 RCTs) | ⨁⨁⨁⨁ | CPNB results in little to no difference in PONV. |
| High | ||||||
∗ The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CPNB: continuous peripheral nerve block, MD: mean difference, OR: odds ratio; PONV: postoperative nausea and vomiting, RCT: randomized controlled trial, RR: relative risk.
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Table 3.
| Outcomes |
Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with epidural analgesia | Risk with CPNB | |||||
| Pain assessed with: Numeric rating scale | The mean pain score was 1.8 | MD 0.45 higher (0.13 lower to 1.04 higher) | - | 375 (8 RCTs) | ⨁⨁◯◯ | The evidence suggests that CPNB results in little to no difference in pain. |
| Low†,‡ | ||||||
| Opioid consumption assessed with: Oral morphine equivalents | The mean opioid consumption was 35.4 mg | MD 16.13 mg higher (0.10 lower to 32.36 higher) | - | 342 | ⨁⨁◯◯ | The evidence suggests epidural analgesia slightly decreases opioid consumption compared to CPNB. |
| (8 RCTs) | Low†,‡ | |||||
| Length of hospital stay assessed with: days | The mean length of hospital stay was 5.7 days | MD 0.78 days higher (0.27 higher to 1.29 higher) | - | 399 (8 RCTs) | ⨁⨁◯◯ | The evidence suggests epidural analgesia decreases length of hospital stay compared to CPNB. |
| Low†,‡ | ||||||
| Postoperative nausea and vomiting | 103 per 1,000 | 104 per 1,000 (60 to 175) | OR 1.02 (0.56 to 1.86) | 265 (5 RCTs) | ⨁⨁⨁◯ | CPNB likely results in little to no difference in PONV compared to epidural analgesia. |
| Moderate† | ||||||
* The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CPNB: continuous peripheral nerve block, MD: mean difference, OR: odds ratio, PONV: postoperative nausea and vomiting, RCT: randomized controlled trial, RR: relative risk.
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download