Introduction
Materials and Methods
1. Case selection and clinicopathological analysis
2. TMA construction and immunohistochemical evaluation
3. Genetic analyses using acute myeloid leukemia cases based on the Cancer Genome Atlas Network data
4. Statistical analysis
Results
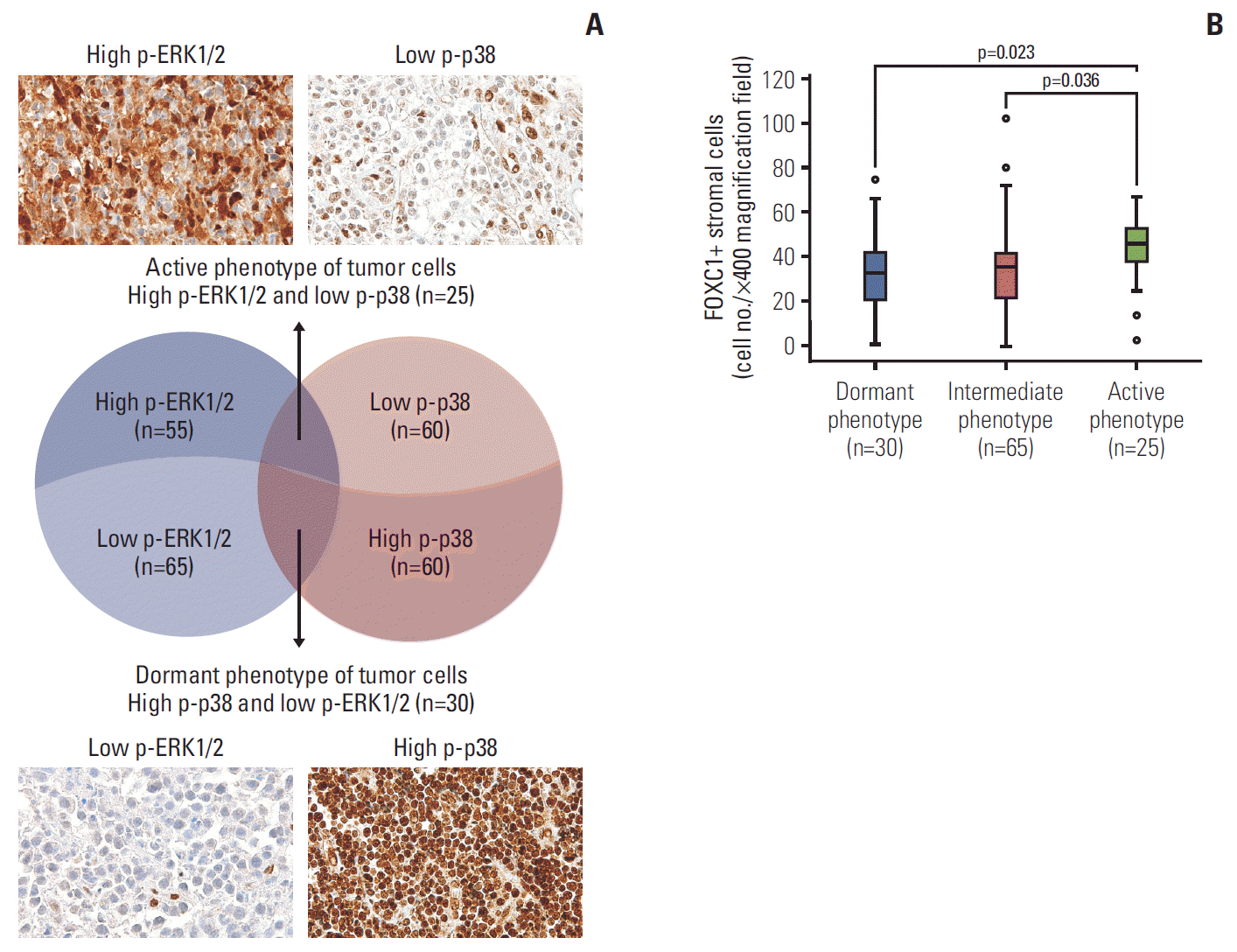
1. Association of FOXC1+ stromal cells in the tumor microenvironment with tumor dormancy and activation
Fig. 1.
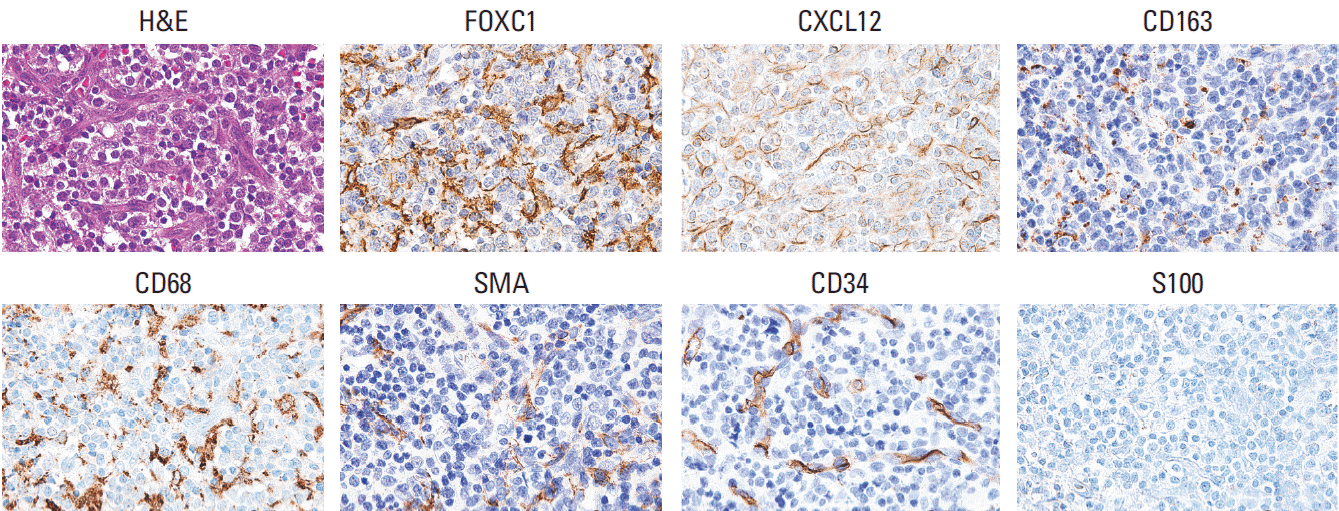
Fig. 2.
2. Status of FOXC1+ stromal cells in the tumor microenvironment with respect to clinicopathological variables
Fig. 3.
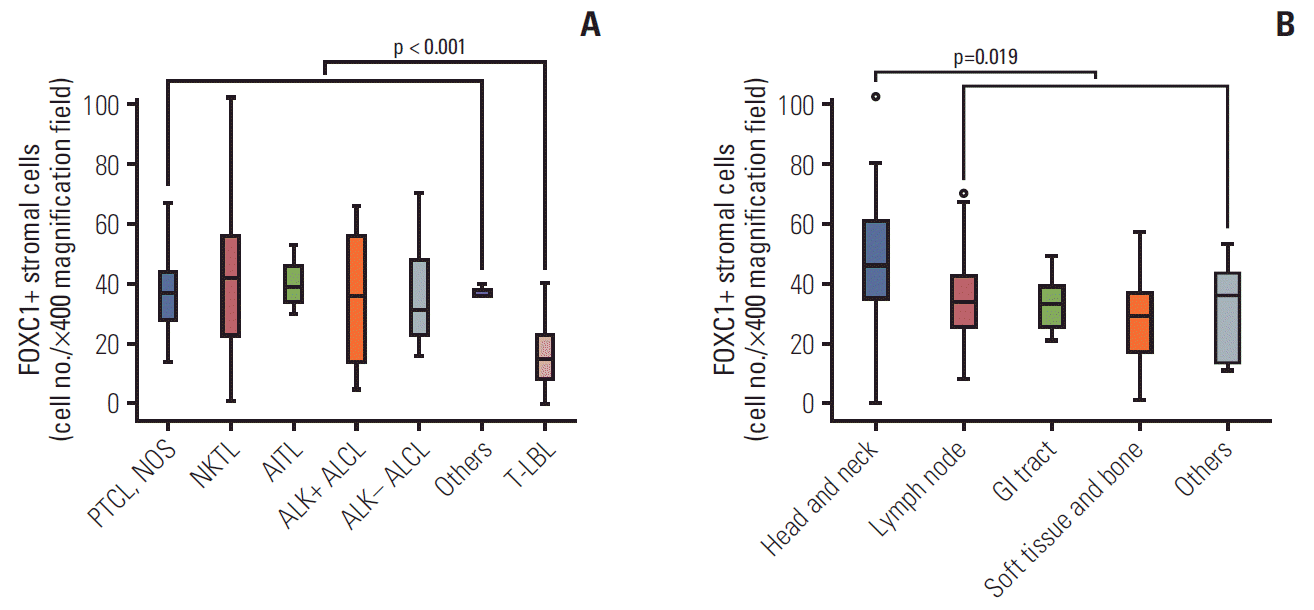
Table 1.
| Clinical feature | No. (%) |
FOXC1+ stromal cell infiltration (number) |
FOXC1+ stromal cell infiltration (low and high) |
|||
|---|---|---|---|---|---|---|
| Mean±SD | p-value | Low (n=95) | High (n=25) | p-value | ||
| Age (yr) | ||||||
| < 60 | 78 (65.0) | 37.59±19.40 | 0.354 | 59 (62.1) | 19 (76.0) | 0.195 |
| ≥ 60 | 42 (35.0) | 34.38±15.09 | 36 (37.9) | 6 (24.0) | ||
| Sex | ||||||
| Male | 86 (71.7) | 34.08±16.50 | 0.020* | 71 (74.7) | 15 (60.0) | 0.146 |
| Female | 34 (28.3) | 42.50±20.39 | 24 (25.3) | 10 (40.0) | ||
| Subtype | ||||||
| PTCL, NOS | 35 (29.2) | 35.17±12.06 | 0.003* | 32 (33.7) | 3 (12.0) | 0.008* |
| NKTL | 39 (32.5) | 42.23±22.53 | 24 (25.3) | 15 (60.0) | ||
| AITL | 12 (10.0) | 40.42±7.84 | 10 (10.5) | 2 (8.0) | ||
| ALCL, ALK+ | 10 (8.3) | 37.00±20.97 | 6 (6.3) | 4 (16.0) | ||
| ALCL, ALK- | 10 (8.3) | 35.50±15.53 | 9 (9.5) | 1 (4.0) | ||
| T-LBL | 11 (9.2) | 16.00±11.82 | 11 (11.6) | 0 | ||
| Others | 3 (2.5) | 37.33±2.31 | 3 (3.2) | 0 | ||
| Primary site | ||||||
| Lymph node | 63 (52.5) | 34.97±13.18 | 0.018* | 56 (58.9) | 7 (28.0) | 0.001* |
| Head and neck | 31 (25.8) | 45.07±25.12 | 16 (16.8) | 15 (60.0) | ||
| GI tract | 8 (6.7) | 33.13±9.43 | 7 (7.4) | 1 (4.0) | ||
| Soft tissue and bone | 11 (9.2) | 27.00±16.32 | 10 (10.5) | 1 (4.0) | ||
| Others | 7 (5.8) | 30.57±17.60 | 6 (6.3) | 1 (4.0) | ||
| LDH levela) | ||||||
| Normal | 33 (39.3) | 29.85±15.64 | 0.013* | 28 (42.4) | 5 (27.8) | 0.259 |
| Elevated | 51 (60.7) | 40.00±19.08 | 38 (57.6) | 13 (72.2) | ||
| BM involvementa) | ||||||
| Absent | 67 (69.8) | 36.93±19.27 | 0.495 | 53 (66.2) | 14 (87.5) | 0.136 |
| Present | 29 (30.2) | 34.17±14.87 | 27 (33.8) | 2 (12.5) | ||
| Ann-Arbor stagea) | ||||||
| I-II | 24 (26.7) | 32.54±22.87 | 0.275 | 18 (25.4) | 6 (31.6) | 0.586 |
| III-IV | 66 (73.3) | 37.38±16.63 | 53 (74.6) | 13 (68.4) | ||
| IPI scorea) | ||||||
| 0-2 | 55 (60.4) | 32.07±19.63 | 0.016* | 44 (61.1) | 11 (57.9) | 0.799 |
| 3-5 | 36 (39.6) | 41.53±15.06 | 28 (38.9) | 8 (42.1) | ||
Values are presented as number (%) unless otherwise indicated. FOXC1, forkhead box C1; NK, natural killer; SD, standard deviation; PTCL, NOS, peripheral T cell lymphoma, not otherwise specified; NKTL, extranodal natural killer/T cell lymphoma; AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; T-LBL, precursor T lymphoblastic leukemia/lymphoma; GI, gastrointestinal; LDH, lactate dehydrogenase; BM, bone marrow; IPI, International Prognostic Index.
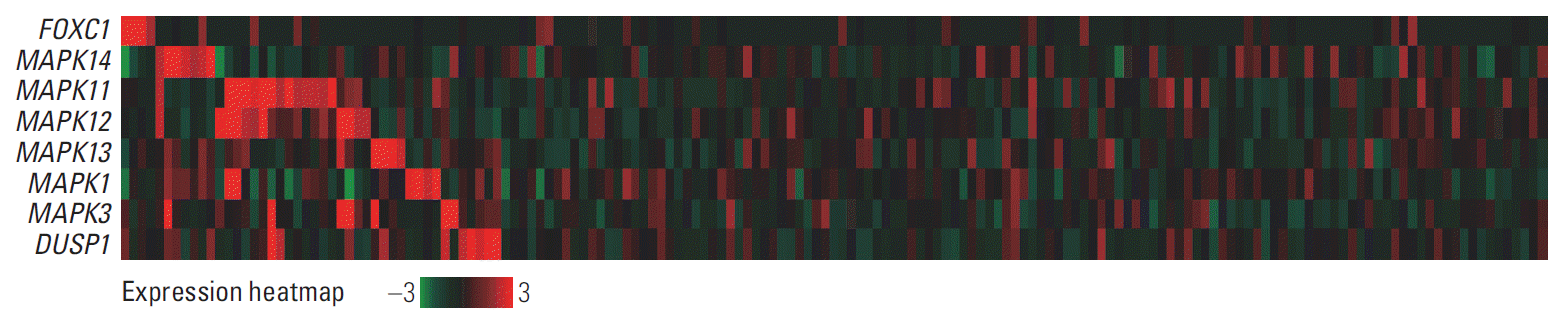
3. Comparison of FOXC1, p38 MAPK, and ERK MAPK mRNA expression levels in AML cases
Fig. 4.
4. Prognostic implications of FOXC1+ stromal cells within the T or NK cell lymphoma microenvironments
Fig. 5.
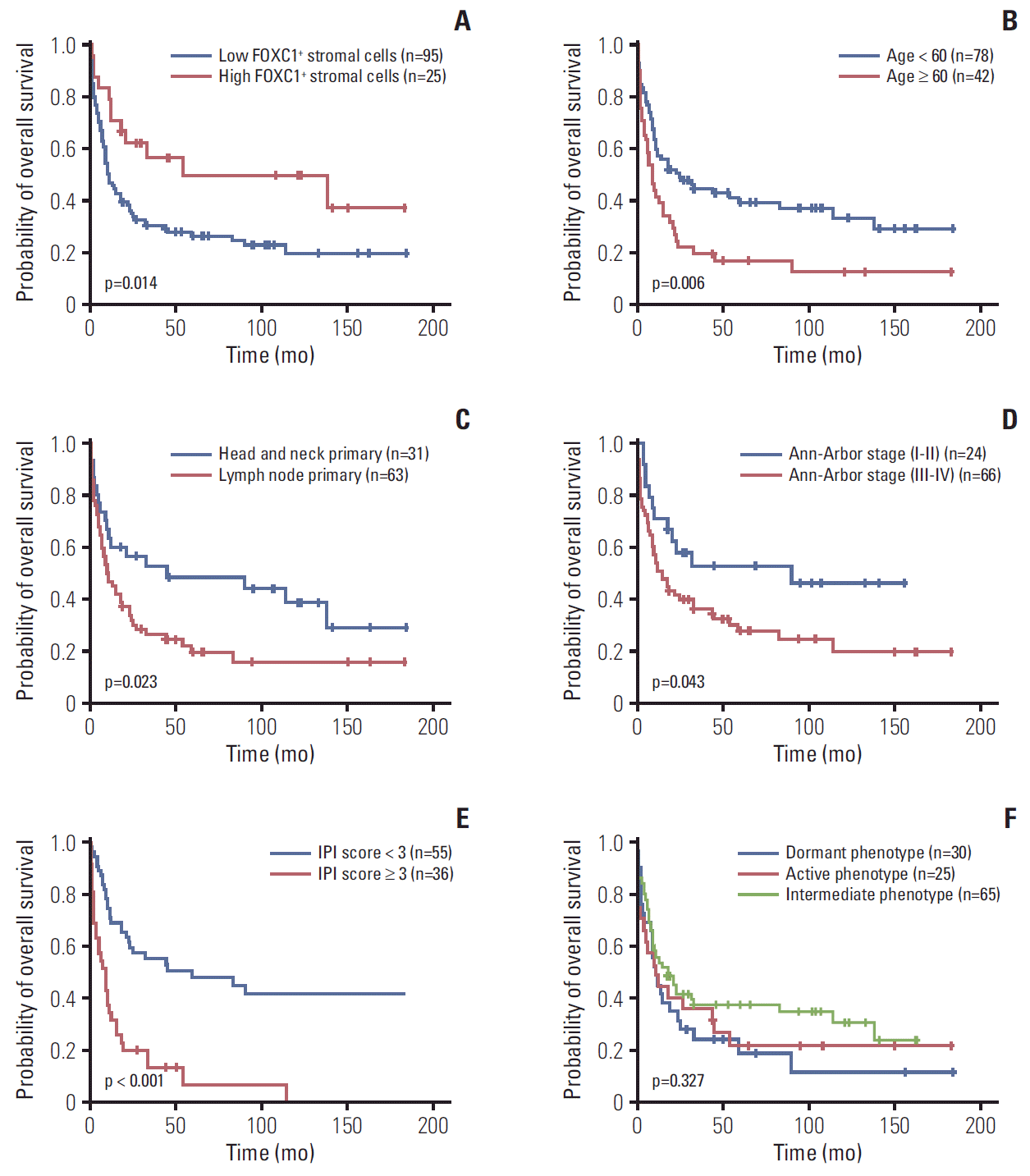
Table 2.
| Variable |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥ 60 yr vs. < 60 yr) | 1.8 | 1.2-2.8 | 0.007* | - | - | - |
| Sex (female vs. male) | 1.5 | 0.9-2.3 | 0.102 | - | - | - |
| Subtype (vs. PTCL, NOS) | ||||||
| NKTL | 0.7 | 0.4-1.2 | 0.180 | - | - | - |
| AITL | 1.2 | 0.6-2.4 | 0.665 | - | - | - |
| ALK+ ALCL | 0.1 | 0.03-0.6 | 0.008* | - | - | - |
| ALK– ALCL | 2.0 | 1.0-4.2 | 0.064 | - | - | - |
| T-LBL | 0.9 | 0.4-1.9 | 0.754 | - | - | - |
| Others | 0.3 | 0.04-2.4 | 0.267 | - | - | - |
| Tumor sites (vs. lymph node) | ||||||
| Head and neck | 0.5 | 0.3-0.9 | 0.029* | - | - | - |
| GI tract | 1.3 | 0.6-2.9 | 0.509 | - | - | - |
| Soft tissue and bone | 0.3 | 0.1-0.8 | 0.021* | - | - | - |
| Others | 0.8 | 0.3-2.0 | 0.633 | - | - | - |
| LDHa) (elevated vs. normal) | 1.3 | 0.7-2.2 | 0.375 | - | - | - |
| BM involvementa) (present vs. absent) | 1.1 | 0.7-1.9 | 0.646 | - | - | - |
| Ann-Arbor stagea) (III-IV vs. I-II) | 1.9 | 1.0-3.6 | 0.049* | - | - | - |
| IPI scorea) (3-5 vs. 1-2) | 3.4 | 2.0-5.6 | < 0.001* | 3.5b) | 2.1-6.0 | < 0.001* |
| Phenotype (vs. active phenotype) | ||||||
| Dormant phenotype | 1.1 | 0.6-2.0 | 0.812 | - | - | - |
| Intermediate phenotype | 0.8 | 0.4-1.3 | 0.331 | - | - | - |
| FOXC1+ stromal cells (low vs. high) | 2.1 | 1.1-3.9 | 0.014* | 1.9 | 1.0-3.8 | 0.059 |
NK, natural killer; HR, hazard ratio; CI, confidence interval; PTCL, NOS, peripheral T cell lymphoma, not otherwise specified; NKTL, extranodal natural killer/T cell lymphoma; AITL, angioimmunoblastic T cell lymphoma; ALK, anaplastic lymphoma kinase; ALCL, anaplastic large-cell lymphoma; T-LBL, precursor T lymphoblastic leukemia/lymphoma; GI, gastrointestinal; LDH, lactate dehydrogenase; BM, bone marrow; IPI, International Prognostic Index; FoxC1, forkhead box C1.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download