INTRODUCTION
The cyclic AMP (3′, 5′-cyclic adenosine monophosphate, cAMP) is a second messenger formed by adenylate cyclase following the stimulation of cells by many neurotransmitters and hormones. cAMP activates effector molecules such as cAMP-dependent protein kinase (protein kinase A, PKA), exchange protein directly activated by cAMP, and cyclic nucleotide-gated ion channels. The effector molecules control a variety of physiological phenomena, including energy metabolism, gene expression, differentiation, proliferation, and apoptosis.
1 Abnormal alteration in cAMP signaling can result in various diseases, including endocrine disorders and cancer,
2 and cAMP signaling is a potential target for developing new therapy for related diseases.
3
Genomic DNA continuously encounters DNA injuries caused by various exogenous or endogenous insults, such as ionizing radiation, chemicals, and stalled replication. When DNA damage is not repaired correctly by cellular repair systems, they can cause mutations, chromosomal aberrations, aneuploidy, and cell death.
4 The misrepaired DNA may induce cell death and numerous diseases, such as cancer and neurological disease.
5 The most serious DNA injuries are DNA double-strand breaks (DSBs), and DSBs are fixed mainly by non-homologous end joining (NHEJ) across the cell cycle and homologous recombination at the S/M phase of cell cycle.
6
cAMP signaling was found to be involved in DNA repair and the development of drug resistance to chemotherapeutic agents,
7 to enhance the repair of cyclobutane pyrimidine dimers induced by ultraviolet (UV) radiation
8 and to increase the levels of enzymes for base excision repair.
9 cAMP signaling was also described to inhibit the base excision repair of 8-oxo-deoxyguanosine induced by γ-ray irradiation
10 and the NHEJ repair of DNA DSBs resulted from γ-radiation.
11 However, the effect of cAMP on DNA repair is not consistent in the literature, and the mechanism underlying the different effects of cAMP remains unclarified. The different effects of cAMP signaling on DNA repair may result from the differences in factors such as DNA damaging agents, type of DNA damage, DNA repair mechanisms, and cell types. This study aimed to investigate the different mechanisms by which cAMP signaling modulates DNA repair by analyzing the cell-type-specific differences in the modulation of DNA repair by cAMP signaling following γ-ray irradiation.
METHODS
Cells, cell culture, and reagents
Human malignant melanoma cells (SK-MEL-28, male; SK-MEL-2, sex unknown), uterine cervical cancer cells (HeLa, female) and non-small cell lung cancer cells (A549, male; H1299, sex unknown) were obtained from the Korea Cell Line Bank (Seoul, Korea). SiHa human cervical cancer cells (female) were a gift of Dr. HD Youn (Seoul National University, Seoul, Korea). HeLa, SiHa and H1299 cells were grown in Dulbecco's modified Eagle's medium (DMEM), SK-MEL-2 and A549 cells were cultured in RPMI 1640, and SK-MEL-28 cells were cultured in Minimum Essential Medium (MEM). All the media contained 10% fetal bovine serum (Welgene, Gyeongsan, Korea) and 100 units/mL of penicillin/streptomycin (Welgene), and the cells were incubated at 37°C in a 5% CO2 incubator.
Prostaglandin E2 (PGE2) was purchased from Cayman Chemical (Ann Arbor, MI, USA), and okadaic acid was obtained from Tocris Bioscience (Bristol, UK). Isoproterenol, H-89, dimethyl sulfoxide and 4′, 6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N6-phenyladenosine-3′, 5′-cyclic monophosphate (6-Phe-cAMP) was purchased from the Biological Life Science Institute (Bremen, Germany), and bovine serum albumin was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Transfection and western blot analysis
Cells were transfected with expression plasmids using Lipofectamine 3000 (Invitrogen, CA, USA), and were exposed to γ-rays (5 Gy) as previously described.
11 Antibodies against phosphorylated cAMP response element bind protein (p-CREB, Ser139), phosphorylated ataxia telangiectasia-mutated protein (p-ATM, Ser1981), and the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) were obtained from Santa Cruz Biotechnology. Antibodies against p-H2AX (Ser133), phosphorylated PKA substrates, cleaved poly (ADP-ribose) polymerase (PARP), and cleaved caspase-9 were purchased from Cell Signaling Technology (Beverly, MA, USA). An antibody against β-actin was purchased from Sigma-Aldrich, and antibodies against DNA-PKcs phosphorylated at Thr2609 (T2609) and Ser2056 (S2056) were purchased from Abcam (Cambridge, UK). The proteins were visualized using enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA), and the blot images were recorded using the LAS-3000 luminescent image analyzer (Fuji, Tokyo, Japan). The densities of the visualized bands were analyzed using Multi Gauge v.2.3 software (Olympus, Tokyo, Japan), and the band densities were expressed as ratios of the corresponding control densities.
Analysis of NHEJ using fluorescent reporter plasmids and immunofluorescence microscopy
Analysis of NHEJ repair using the NHEJ reporter plasmid
12 and immunofluorescence analysis of DNA-ligase IV and X-ray repair cross-complementing protein 4 (XRCC4) were carried out as described previously.
11
Statistical analysis
All of the experiments were repeated at least three times independently, and the data were expressed as means and standard error. Statistical significance of the difference was analyzed using the nonparametric Mann-Whitney U test, and a P value ≤ 0.05 was considered statistically significant.
DISCUSSION
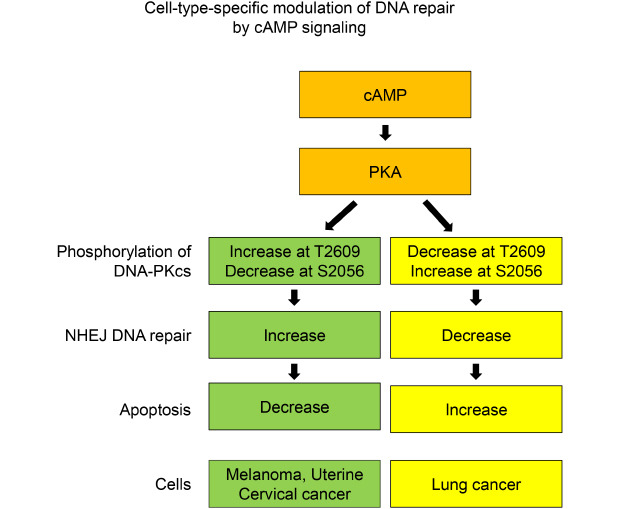
The present study aimed to investigate whether cAMP signaling modulates DNA repair differently depending on the cell type, and we found that cAMP signaling modulates the NHEJ repair of DNA DSBs induced by γ-ray irradiation in malignant melanoma cells, uterine cervical cancer cells, and lung cancer cells and that the cell type specificity might be mediated by PKA-dependent phosphorylation of DNA-PKcs.
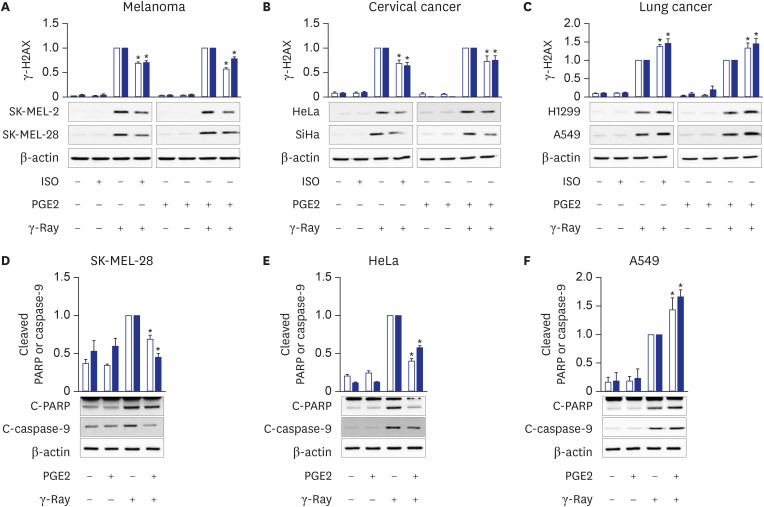
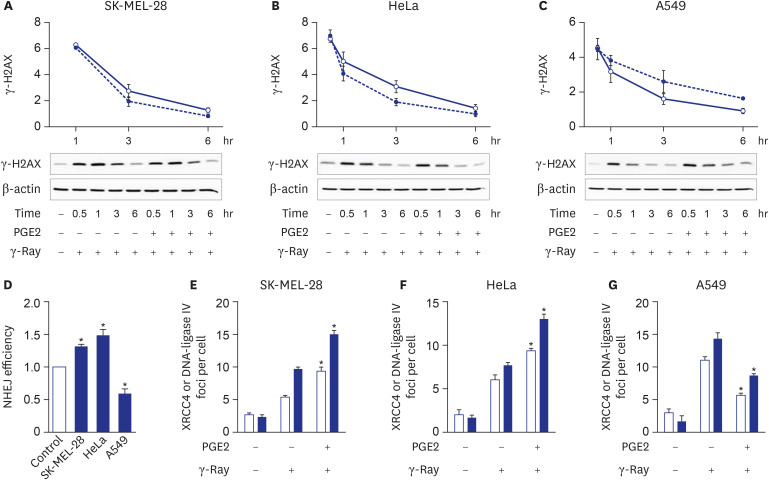
cAMP signaling was found to modulate the NHEJ repair of DNA damage resulted from γ-ray irradiation differently depending upon the cell type. Activation of cAMP signaling of malignant melanoma cells and uterine cervical cancer cells by treatment with PGE2 or isoproterenol decreased DNA damage, as assessed by the γ-H2AX assay following γ-ray irradiation, accelerated the disappearance of γ-H2AX, increased NHEJ efficiency, as determined using fluorescent reporter plasmids, stimulated the recruitment of XRCC4 and DNA-ligase IV, major players in NHEJ, to DSB foci, and decreased apoptosis. By contrast, activation of cAMP signaling in lung cancer cells increased DNA DSBs, delayed the disappearance of γ-H2AX, decreased NHEJ efficiency, reduced the recruitment of XRCC4 and DNA-ligase IV, and augmented apoptosis resulting from exposure to γ-rays.
The present study showed that cAMP signaling modulates NHEJ repair differently depending on the cell type and modulates DNA repair similarly in cells of similar origin, such as lung cancer cells (A549 cells and H1299), melanoma cells (SK-MEL-2 and SK-MEL-28) and cervical cancer cells (HeLa and SiHa). This study compared the NHEJ in the different cells following the same treatment with PGE2 and γ-ray irradiation. The cell-type-specific modulation of cAMP signaling of DNA repair seems to result in the cell-type-specific modulation of apoptosis: cAMP signaling stimulated NHEJ repair and inhibited apoptosis in γ-ray-irradiated melanoma cells and cervical cancer cells, whereas cAMP signaling inhibited NHEJ and stimulated apoptosis resulting from γ-ray irradiation in lung cancer cells. Comparable cell-type-specific regulation by cAMP signaling has already been described in cellular proliferation and apoptosis.
131415 The cell-type-specific regulation by cAMP signaling is speculated to result from cell-type-specific expression of various proteins that function in cAMP signaling, DNA repair, proliferation, and apoptosis. However, more extensive studies on the modulation of NHEJ and other DNA repair mechanisms by cAMP signaling using various cell types are needed to generalize the cell-type-specific modulation of DNA repair by cAMP signaling.
The roles of cAMP signaling in DNA repair have been reported in melanocytes and melanoma cells. Melanocyte-stimulating hormones (MSHs) bind to and activate melanocortin receptors, which activate cAMP signaling in melanoma cells and melanocytes. Activated cAMP signaling leads to the acceleration of nucleotide excision repair to remove DNA photoproducts caused by UV light independent of pigmentation
1617 and cisplatin-induced DNA adducts.
18 Our study indicates that cAMP signaling also stimulates the NHEJ repair of radiation-generated DSBs in melanoma cells, a finding similar to the report that MSH prevented the reactive oxygen species-induced increase in γ-H2AX in melanoma cells.
19 Thus, cAMP signaling seems to stimulate the nucleotide excision repair of UV-induced DNA damage and NHEJ of γ-ray-induced DNA damage in melanocytes and melanoma cells.
Most uterine cervical cancers are associated with infections of high-risk human papillomavirus (HPV).
20 HPV oncogenes, E5, E6 and E7, can stimulate the cyclooxygenase-prostaglandin pathway, which controls inflammation and carcinogenesis, by elevating the expression of cyclooxygenases and cAMP-linked PGE2 receptors, resulting in chronic inflammation and cervical carcinogenesis.
212223 Treatment of cervical cancer cells with lactate was reported to enhance the repair of DNA damage resulted from treatment with neocarzinostatin, doxorubicin and cisplatin.
24 Because lactate receptor (HCAR1 or G-protein-coupled receptor 81, GPR81), was reported to inhibit adenylate cyclase, cAMP signaling might mediate the lactate effects on DNA repair.
25 However, the role of cAMP upon DNA repair in cervical cancer cells remained unclarified. Thus, to our best knowledge, we showed the stimulatory effect of cAMP signaling on the NHEJ repair of DSBs following γ-ray irradiation in cervical cancer cells for the first time. The finding that cAMP inhibits γ-ray-induced apoptosis in cervical cancer cells, together with the report that cAMP signaling inhibits the apoptosis in cervical cancer cells resulting from cisplatin treatment,
26 suggests common mechanisms for cAMP signaling to modulate DNA repair and apoptosis following γ-ray radiation and cisplatin treatment in cervical cancer cells.
cAMP signaling was reported to repress base excision repair
10 and NHEJ repair
11 of DNA damage caused by exposure of lung cancer cells to γ-rays and to augment radiation-generated apoptosis in lung cancer cells.
27 These reports completely agree with and support our finding that cAMP signaling modulates the NHEJ repair of DNA damage and apoptosis following γ-ray irradiation.
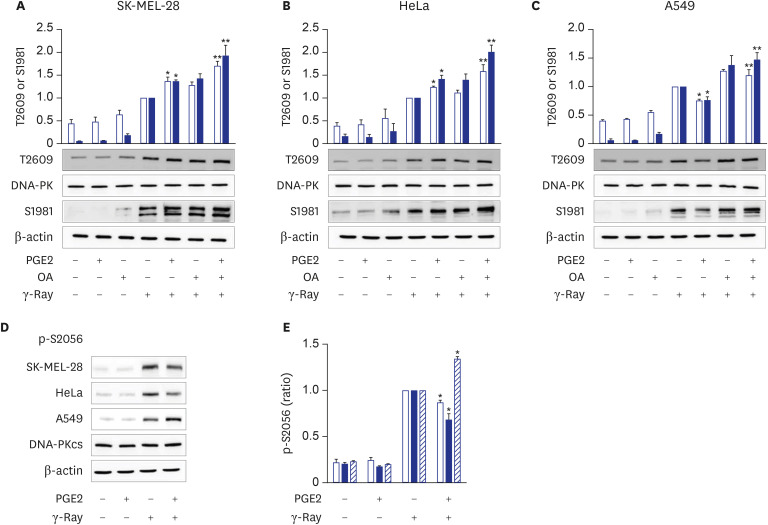
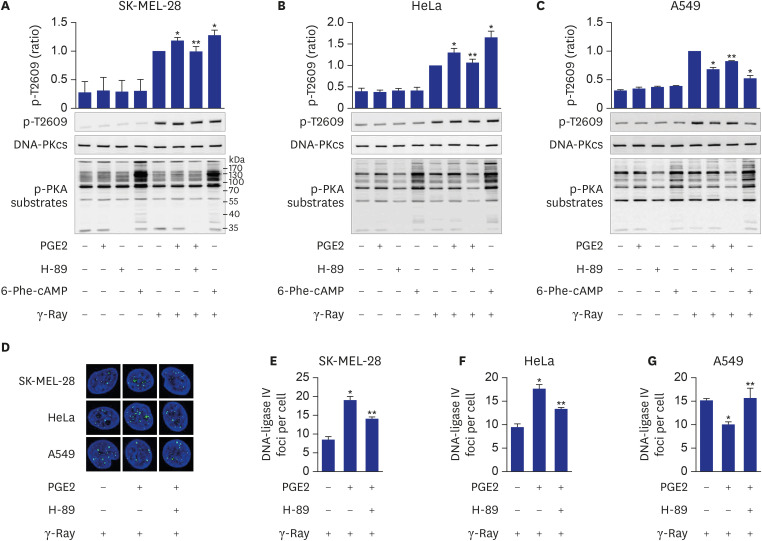
We also found that the cell-type specificity might be mediated by the PKA-dependent phosphorylation of DNA-PKcs. This finding is derived from the results that cAMP differently modulated the phosphorylation of DNA-PKcs following γ-ray irradiation depending on the cell type, that treatment with the PKA-specific activator induced similar phosphorylation patterns to those with PGE2 treatment and that the inhibition of PKA abolished the PGE2 effects on the phosphorylation of DNA-PKcs and the recruitment of DNA-ligase IV and XRCC4 in all the cell types tested.
DNA-PKcs is phosphorylated at multiple threonine and serine residues by DNA-PK itself and many other protein kinases including ATM.
28 The phosphorylation of DNA-PKcs in the ABCDE region containing T2609 and in the PQR region containing S2056 is suggested to affect DNA-PK activity and end processing of damaged DNA, resulting in the alteration of DNA repair activity.
29 Differential phosphorylation of DNA-PKcs recruited to DNA ends functions as a molecular switch controlling end-processing and end ligation in NHEJ.
30 cAMP signaling was reported to modulate NHEJ repair and differential phosphorylation of DNA-PKcs on S2056 and T2609 lung cancer cells exposed to γ-rays.
11 This study showed that cAMP signaling modulates the differential phosphorylation of DNA-PKcs at S2056 and T2609 differently depending on the cell type (cervical cancer cells, lung cancer cells, and melanoma cells), likely resulting in the differential modulation of NHEJ repair activity depending upon the cell type. This study also indicated that PKA mediates the cell-type-specific modulation by cAMP of the differential phosphorylation of DNA-PKcs on S2056 and T2609 following exposure to γ-rays. cAMP signaling also inhibited T2609 phosphorylation in PP2A- and ATM-dependent pathways in lung cancer cells as reported previously
11 and increased T2609 phosphorylation in PP2A-independent pathways in cervical cancer cells and melanoma cells.
In summary, the present study showed that cAMP signaling modulates NHEJ repair in cell-type-specific manners following γ-ray irradiation and that PKA mediates the differential phosphorylation of DNA-PKcs at T2609 and S2056 depending on the cell type. In melanoma and uterine cervical cancer cells, PKA activated by cAMP increases the phosphorylation of DNA-PKcs on T2609 with a concomitant decrease in S2056 phosphorylation, which might enhance DNA-PK activity to stimulate NHEJ repair. By contrast, in lung cancer cells, PKA decreases the phosphorylation of DNA-PKcs on T2609 with a concomitant increase in S2056 phosphorylation, which might inhibit DNA-PK activity and delay NHEJ repair.
The finding that cAMP signaling modulates the repair of radiation-induced DNA damage in a cell type- and tissue-specific manner suggests that activity of cAMP signaling might affect the repair of DNA damage caused by radiotherapy to treat in cancer patients. Therefore, therapeutic efficiency of radiotherapy could be improved by modulating the activity of cAMP signaling to inhibit DNA repair of cancer cells or to stimulate DNA repair of normal tissues surrounding metastatic cancer cells.
In conclusion, cAMP signaling modulates the cell-type-specific NHEJ repair of γ-ray-induced DNA DSBs in melanoma cells, uterine cervical cancer cells and lung cancer cells, and these effects may be mediated by PKA-dependent, cell-type-specific differential phosphorylation of DNA-PKcs. This study suggests that the cell type- and tissue-specific modulation of DNA damage repair by cAMP signaling might be applied to improve the therapeutic efficiency of radiation therapy for cancer.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download