| J Rheum Dis. 2015 Aug;22(4):263-268. English. Published online August 25, 2015. https://doi.org/10.4078/jrd.2015.22.4.263 | |
| Copyright © 2015 by The Korean College of Rheumatology | |
|
Jinyoung Moon,1
Nakwon Kwak,1
Jin Lim,1
Dong Jin Go,1
Jae Hyun Lee,1
Jin Kyun Park,1
Eun Bong Lee,1
Yeong Wook Song,1
Jai Il Youn,2
and Eun Young Lee | |
|
1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. | |
|
2Department of Dermatology, National Medical Center, Seoul, Korea. | |
| Received August 04, 2014; Revised September 22, 2014; Accepted September 23, 2014. | |
|
This is a Free Access article, which permits unrestricted non-commerical use, distribution, and reproduction in any medium, provided the original work is properly cited. | |
|
Abstract
| |
|
Nowadays, tumor necrosis factor-α (TNF-α) blockers are used for treatment of rheumatoid arthritis, inflammatory bowel diseases, ankylosing spondylitis, psoriatic arthritis, and psoriasis. Paradoxically, there are some reports on the appearance of psoriasis after administration of TNF-α blockers. Here, we report on a patient with monoarthritis in a knee joint who experienced psoriasis after TNF-α blocker therapy (adalimumab and etanercept). Oral medication was not a treatment option due to patient intolerance; thus, we tried ustekinumab, an anti-interleukin (IL)-12/23 monoclonal antibody used for treatment of psoriasis. Following ustekinumab injection, psoriatic skin lesions and joint symptoms were much improved. However, in the following period, joint pain and swelling became aggravated and synovial fluid cytokine levels including IL-6 and IL-17 were elevated. The treatment was changed to tocilizumab, a humanized monoclonal antibody against IL-6 receptor. After injection, knee joint swelling rapidly subsided without worsening of psoriatic skin lesions. |
|
Keywords: Tumor necrosis factor-α blocker; Monoarthritis; Psoriasis; Ustekinumab; Tocilizumab |
|
|
INTRODUCTION
|
The tumor necrosis factor (TNF) family has anti-tumor and immune regulating properties. There are two distinct members of the TNF family, TNF-α and β. TNF-α is a regulator of apoptosis and proinflammatory cytokines with pleiotropic actions [1]. Thus, dysregulation of TNF-α is related to the pathophysiology of various inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis, psoriatic arthritis, and psoriasis. The efficacy and safety of TNF-α blockers have been proven by several randomized controlled trials in psoriasis patients. However, because of the increased use of TNF-α blockers, side effects from these drugs have been reported. The adverse effects include opportunistic infections, reactivation of tuberculosis, various cutaneous side effects, lupus like reactions, demyelinating disease, heart failure and lymphoma [2, 3, 4].
Although the administration of a TNF-α blocker is indicated in the treatment of psoriasis, some case reports have shown that TNF-α blocker might induce psoriasis flare-ups and changes in morphology [5, 6, 7, 8].
A recent randomized controlled trial of IL-12/23 monoclonal antibody ustekinumab showed a favorable response for the treatment of psoriasis and psoriatic arthritis [9, 10]. Tocilizumab, an interleukin (IL)-6 receptor blockade, is used to treat disease modifying anti-rheumatic drugs (DMARDs) refractory rheumatoid arthritis (RA), and a favorable response has been observed without using concomitant methotrexate [11]. Here, we report on a refractory monoarthritis patient who previously had no skin lesions, but experienced psoriatic skin lesions after administration of a TNF-α blocker. The psoriasis of this patient responded to treatment with IL-12/23 blockade; however, the arthritis responded well to treatment with IL-6 receptor blockade.
|
CASE REPORT
|
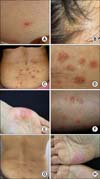
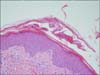
A 53-year-old oriental woman visited a rheumatology clinic for right knee swelling and pain. She had a history of hypertension. She had never smoked. Her occupation is a housewife. She did not have a family history of spondyloarthritis. The patient experienced right knee swelling and pain after mountain climbing in the spring of 2009. Her symptoms were wax and wane. After several years of observation, the patient finally visited Seoul National University Hospital in February 2011. There was no inflammatory back pain during that period. For a diagnosis, a knee magnetic resonance imaging (MRI) was done which revealed diffuse synovial hypertrophy (Figure 1A). A diagnostic synovectomy was done for the knee arthritis, and histopathologically, there was chronic inflammation, lymphoplasmacytic infiltration, lymphoid follicles and synovial hyperplasia suggesting RA (Figure 2). At that time, rheumatoid factor, anti-cyclic citrullinated protein antibody and HLA-B27 were all negative. In a simple X-ray, there was no sacroiliitis. We tentatively diagnosed the patient with seronegative rheumatoid arthritis when taking into consideration the age, sex, hypertrophic synovial membrane and pathology, even though her case did not match the classification criteria. We used low dose glucocorticoids and methotrexate, leflunomide, or sulfasalazine. However, those medications lead to a loss of hair, abdominal pain, nausea and generalized edema; thus, we decided to stop all medications. Adalimumab injection was started in July 2012 and continued biweekly for 8 weeks, after which, the joint swelling in her right knee was reduced. However, after the 8th injection of adalimumab, a pustular skin rash appeared on her face, scalp, and trunk. We stopped the adalimumab injection because the newly appeared skin lesions were considered to be drug-related (Figure 3A and 3B). Thereafter, there was no more exacerbation of the skin rash after discontinuing the medication, but it still persisted. Because of the previous improvement in knee swelling after the use of a TNF-α blocker, we tried etanercept as a therapy in January 2013. After 4 injections of etanercept, there were psoriatic skin rashes over her whole body (Figure 3C and 3D). Thus, we stopped the medication again. A skin biopsy on her back revealed hyperkeratosis and irregular acanthosis in the epidermis suggesting active psoriasis. A focal decrease in the granular layer and inflammatory cells, especially lymphocyte infiltration at the dermis, was observed (Figure 4). Her skin lesions eventually developed to a palmar and shin pustular type indicating active psoriasis (Figure 3E and 3F).
|
|
|
|
Four weeks later, starting from her last injection of etanercept, we started ustekinumab injections in February 2013 (45 mg, subcutaneously) to control her psoriasis and active synovitis in the right knee. Two weeks later, her knee swelling and pain had been slightly reduced, and then, 2 more injections of ustekinumab were given at 4 and 12 weeks after the 1st injection. Although the psoriatic skin lesions had much improved after the 3rd injection of ustekinumab (Figure 3G and 3H), the right knee synovitis did not respond to the ustekinumab treatment, and the synovial swelling became aggravated after transient improvement.
We examined some cytokine levels from her synovial fluid. Before the ustekinumab injection, the levels of IL-6 and IL-17 in the synovial fluid were elevated compared to the levels 1.5 years prior (Figure 5). After the first ustekinumab injection, the synovial cytokine levels were markedly decreased. However in the following period, joint symptoms became aggravated again, and the levels of IL-6 and IL-17 were elevated simultaneously. After 10 weeks from the third injection of ustekinumab, we started tocilizumab (400 mg, intravenously, monthly), a humanized monoclonal antibody against the IL-6 receptor, to treat the refractory synovitis. Joint swelling of her right knee and tenderness decreased after the 2nd injection of tocilizumab without any exacerbation of skin psoriasis. Tocilizumab was well tolerated and has not shown any skin or arthritis flare-ups.
|
|
DISCUSSION
|
TNF-α blockers are widely used in dermatologic, rheumatic, and autoimmune disorders. The safety of these drugs has been shown by many reports [2, 3, 4]. TNF-α blockers have been used to treat psoriasis. However, paradoxically, psoriasis newly develops de novo after therapy. Several case reports have revealed this phenomenon [5, 7]. This has been shown to be because of the interaction between TNF-α and interferon-α (IFN-α) [7]. This concept explains that plasmacytoid dendritic cells are the main source of IFN-α with increased levels in early psoriatic skin lesions. IFN-α induces the expression of CXCR3 in T cells, and then, T cells migrate to the skin. IFN-α accelerates the secretion of proinflammatory cytokines from myeloid dendritic cells. Thus, IFN-α injection can induce the development or exacerbation of psoriasis [12, 13]. TNF-α blocks IFN-α production through 2 ways. One way is to block the maturation of plasmacytoid dendritic cells. The other way is to block the release of IFN-α [8]. Following these theories, the development and exacerbation of psoriasis can be explained as follows: TNF-α blockers affect the function of TNF-α which regulates IFN-α production.
We tentatively diagnosed that the patient had seronegative rheumatoid arthritis from the synovial biopsy and knee MRI. Other disease criteria, including psoriatic arthritis, did not match her symptoms and signs. We started administering a TNF-α blocker because of the patient's intolerance to oral DMARDs (methotrexate, methylprednisolone, leflunomide, and sulfasalazine). After several injections of this drug, psoriatic skin lesions appeared. Because the skin biopsy pathology was compatible to psoriasis, we tried ustekinumab, a monoclonal antibody that binds to the shared p40 subunit of the IL-12/23 monoclonal antibody. IL-12/23 is critical in the pathophysiology of psoriasis, and ustekinumab is a novel drug in the treatment of psoriasis and psoriatic arthritis [9]. After the injection of ustekinumab, the skin change looked promising. After 12 weeks later, the skin lesions all disappeared. At the time of the 1st ustekinumab injection, a follow-up MRI was done. There was still scattered synovial hypertrophy in the right knee joint cavity. Because the beneficial role of synovectomy for the knee has been clearly shown for inflammatory arthritis, we also considered therapeutic synovectomy. However, at that time, the patient felt better in her joint than before the diagnostic synovectomy, and she was reluctant to undergo surgery. Thus, we just performed medical treatment. We did an analysis of the cytokines in the joint fluid including IL-6 and 17 (Bio-Plex 200&Luminex; Bio-Rad, Hercules, CA, USA) to further establish a management plan. Before the ustekinumab injection, the cytokine levels were elevated to 60,655.64 (IL-6)/78.51 (IL-17) pg/dL. After the first injection, the synovial cytokine levels were greatly decreased (Figure 5). IL-17 production has been observed in RA synovial tissue, and IL-6 is also known to be abundant in both the synovial fluid and sera of RA patients [11, 14, 15]. The patient showed transient improvement in synovitis after IL-12/23 receptor blockade, and arthritis flare-up accompanied the increased level of IL-6 (20,878.86 pg/dL) and IL-17 (68.70 pg/dL). IL-6 affects the function of inflammatory cells including neutrophils, T cells, B cells, monocytes, and osteoclasts which induce joint destruction and active arthritis. Tocilzumab, a monoclonal antibody against the IL-6 receptor could be a good treatment choice in this case because arthritis flare could be associated with increased IL-6 production in the inflamed synovium [11]. Her initial diagnosis was equivocal because of unmatched classification criteria; however, through a retrospective review, we ascertained that the exceptional response to tocilizumab was a key factor in her disease. We also checked other inflammatory cytokines such as TNF-α, IL-1, and IFN-γ but did not see any significant change according to flare-ups (data not shown).
|
SUMMARY
|
TNF-α blockers can promote psoriasis development and flare-ups, and IL-12/23 blockade or IL-6 blockade could be a good alternative treatment for inflammatory arthritis with psoriasis induced after TNF-α blocker treatment. Evaluation of inflammatory cytokines within the involved joint synovial fluid can be used to select the appropriate biological treatment in refractory monoarthritis.
|
Notes
|
CONFLICT OF INTEREST:No potential conflict of interest relevant to this article was reported.
|
References
|
| 1. | Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992;10:411–452. |
| 2. | Colombel JF, Loftus EV Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohns disease: the Mayo clinic experience in 500 patients. Gastroenterology 2004;126:19–31. |
| 3. | Harriman G, Harper LK, Schaible TF. Summary of clinical trials in rheumatoid arthritis using infliximab, an anti-TNFalpha treatment. Ann Rheum Dis 1999;58 Suppl 1:I61–I64. |
| 4. | Genovese MC, Bathon JM, Fleischmann RM, Moreland LW, Martin RW, Whitmore JB, et al. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 2005;32:1232–1242. |
| 5. | Goiriz R, Daudén E, Pérez-Gala S, Guhl G, García-Díez A. Flare and change of psoriasis morphology during the course of treatment with tumour necrosis factor blockers. Clin Exp Dermatol 2007;32:176–179. |
| 6. | Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum 2005;52:2513–2518. |
| 7. | Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat 2009;20:100–108. |
| 8. | Cuchacovich R, Espinoza CG, Virk Z, Espinoza LR. Biologic therapy (TNF-alpha antagonists)-induced psoriasis: a cytokine imbalance between TNF-alpha and IFN-alpha? J Clin Rheumatol 2008;14:353–356. |
| 9. | Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–1674. |
| 10. | Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–640. |
| 11. | Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–997. |
| 12. | Gilliet M, Conrad C, Geiges M, Cozzio A, Thürlimann W, Burg G, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol 2004;140:1490–1495. |
| 13. | Ladoyanni E, Nambi R. Psoriasis exacerbated by interferon-alpha in a patient with chronic myeloid leukemia. J Drugs Dermatol 2005;4:221–222. |
| 14. | Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 2008;4:767–775. |
| 15. | Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 1999;42:963–970. |




 ePub
ePub Citation
Citation Print
Print