INTRODUCTION
Recent advances in the understanding of the pathogenesis of psoriasis have resulted in the introduction of new biological agents that can inhibit the action of specific molecules related to the pathogenesis of psoriasis
1. Ustekinumab is a human monoclonal antibody that targets the p40 subunit of interleukin (IL)-12/IL-23, thus inhibiting the IL-1/IL-17 axis in psoriasis
2. The long-term efficacy and safety of ustekinumab in the treatment of psoriasis are well established and long-term continuous treatment has been reported to be the most effective treatment option for ustekinumab treatment
345. Studies investigating the outcomes of ustekinumab treatment in real clinical practice also support the effectiveness and safety of long-term continuous ustekinumab treatment
345678. Studies on drug survival in psoriasis treatment reported that ustekinumab had better retention rates than anti-tumor necrosis factor agents, and the rate of adverse events was also favorable
67910.
In contrast with conventional systemic agents or phototherapy, newly introduced biologic agents can be used continuously. Moreover, it has been reported that the continuous use of biologic agents results in greater and sustained therapeutic effects
3. In addition, the lack of cumulative toxicity supports the continuous use of biologic agents in the treatment of psoriasis
3411. However, there are some patients who use biologic agents intermittently and repetitively. When choosing treatment modalities for psoriasis, the main determining factors are their effectiveness and any adverse events. However, other factors such as comorbidities, cost, and time required for treatment also influence patients' choice.
To determine which factors lead to intermittent and repetitive ustekinumab treatment instead of long-term continuous treatment, we investigated the characteristics of psoriasis patients who were treated with ustekinumab intermittently or repetitively and described the importance of economic factors in ustekinumab treatment.
Go to :

MATERIALS AND METHODS
Patients and data acquisition
We retrospectively enrolled 30 psoriasis patients who discontinued ustekinumab treatment and were followed up for psoriasis treatment. We defined the discontinuation of ustekinumab as discontinued ustekinumab treatment for more than 6 months after the last injection of ustekinumab. We defined a treatment period as the period in which the main treatment modalities are identical (
Fig. 1). The 30 patients received 77 treatments periods during the study period, of which 52 were ustekinumab treatments. In addition, 8 periods of biologic agents other than ustekinumab, 7 periods of cyclosporine, 6 periods of methotrexate, 2 periods of acitretin, and 2 periods of phototherapy were included in the study. At the end of the study, 15 patients were undergoing ustekinumab treatment. This study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB no. B-1603-338-102).
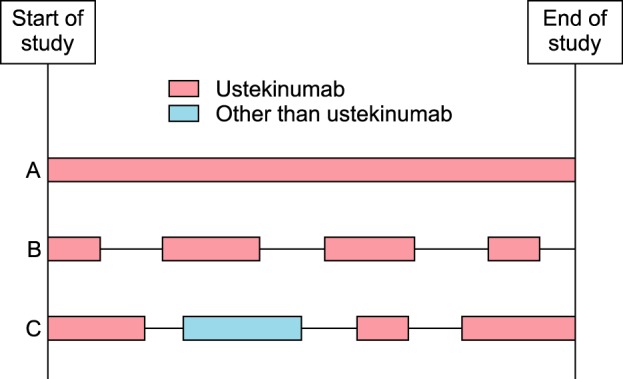 | Fig. 1A conceptual diagram of the treatment period. In patient A, the treatment of ustekinumab was continuous (one treatment period during the study). In patient B, four ustekinumab treatment periods during the study were observed according to severity of psoriasis. In patient C, after the discontinuation of initial ustekinumab treatment, the patient was treated intermittently and repetitively with ustekinumab or other systemic agents and phototherapy according to severity of psoriasis. In this study, we enrolled the patients who discontinued the first ustekinumab treatment and were followed up for the treatment of psoriasis such as patient B and patient C.
|
We collected data regarding the clinical characteristics of the patients and information on psoriasis treatment from medical records. The clinical characteristics reviewed were age, gender, body mass index, duration of psoriasis, comorbidity, biological agent naivety, age at start of ustekinumab treatment, and previous treatments for psoriasis. The data collected relating to the treatment period were psoriasis area and severity index (PASI) score at each visit, dose of ustekinumab, number of injections of ustekinumab, duration of ustekinumab treatment, method of payment for ustekinumab treatment, and reason for treatment discontinuation. Finally, to assess the effectiveness of ustekinumab treatment, we calculated the 75% and 90% reduction of PASI score from baseline (PASI75 and PASI90, respectively), as well as the time taken to achieve PASI75.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney test and paired t-test for comparing continuous variables. Categorical variables were analyzed using the chi-square test, Fisher's exact test and McNemar-Bowker test. Results were expressed as the mean±standard deviation and a p-value of <0.05 was considered statistically significant. Data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
Go to :

RESULTS
The characteristics of patients and ustekinumab treatment period
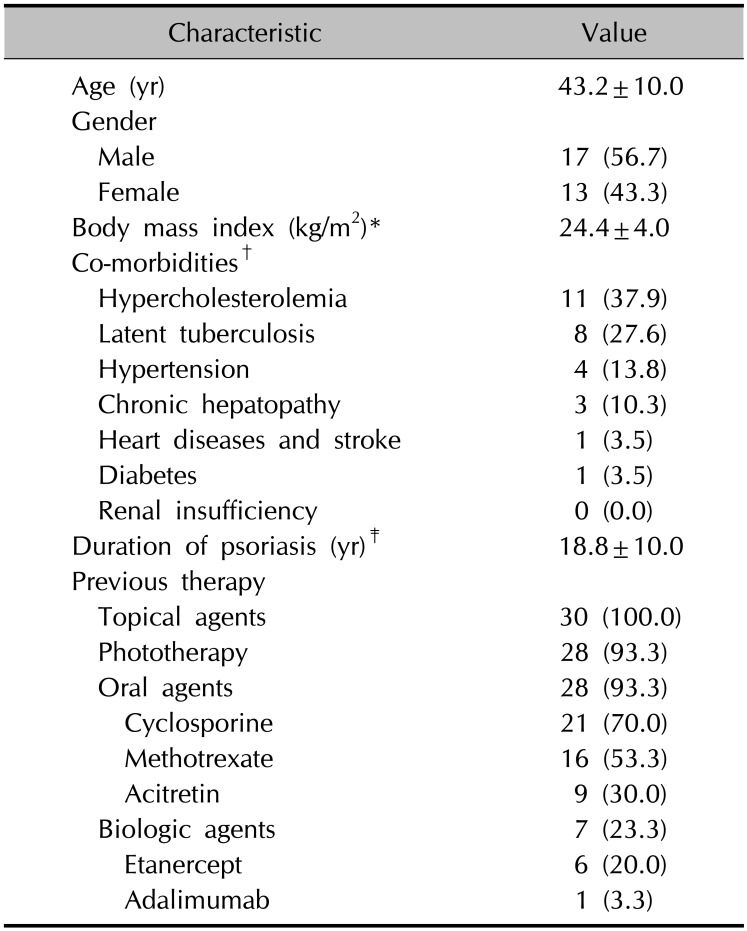
Of the 77 treatment periods in the 30 psoriasis patients, the number of ustekinumab treatment periods was 52 (67.5%). Sixteen patients had ustekinumab treatment periods twice or more, with 12 patients using ustekinumab twice, three patients three times, and one patient five times. At first treatment with ustekinumab, the mean age of the patients was 43.2±9.98 years and the duration of psoriasis 18.8±10.0 years (
Table 1). Among the 30 patients, hypercholesterolemia was found in 11 (37.9%) patients and was the most common comorbidity, followed by latent tuberculosis (8 patients, 27.6%). With respect to previous therapy, 28 (93.3%) of the patients had undergone phototherapy, and 28 (93.3%) had received oral agents before the ustekinumab treatment. Twenty-three (76.7%) patients were biologics-naïve, and the remaining seven (23.3%) had experienced treatment with biologic agents, six treated with etanercept and one with adalimumab.
Table 1
Baseline demographics and characteristics of enrolled patients (n=30)
|
Characteristic |
Value |
|
Age (yr) |
43.2±10.0 |
|
Gender |
|
|
Male |
17 (56.7) |
|
Female |
13 (43.3) |
|
Body mass index (kg/m2)*
|
24.4±4.0 |
|
Co-morbidities†
|
|
|
Hypercholesterolemia |
11 (37.9) |
|
Latent tuberculosis |
8 (27.6) |
|
Hypertension |
4 (13.8) |
|
Chronic hepatopathy |
3 (10.3) |
|
Heart diseases and stroke |
1 (3.5) |
|
Diabetes |
1 (3.5) |
|
Renal insufficiency |
0 (0.0) |
|
Duration of psoriasis (yr)‡
|
18.8±10.0 |
|
Previous therapy |
|
|
Topical agents |
30 (100.0) |
|
Phototherapy |
28 (93.3) |
|
Oral agents |
28 (93.3) |
|
Cyclosporine |
|
|
Methotrexate |
16 (53.3) |
|
Acitretin |
9 (30.0) |
|
Biologic agents |
7 (23.3) |
|
Etanercept |
6 (20.0) |
|
Adalimumab |
1 (3.3) |

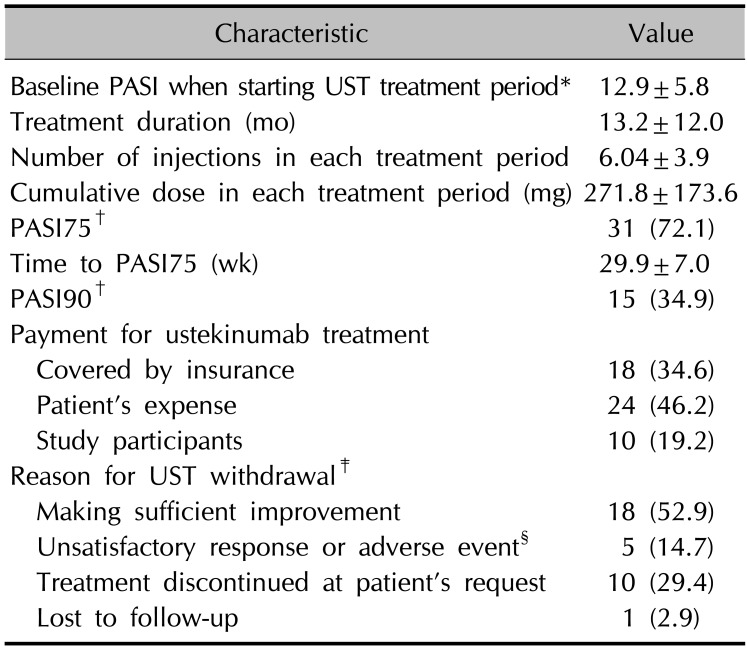
The mean baseline PASI score of all ustekinumab treatment period was 12.9±5.80 and the mean duration of the ustekinumab treatment periods was 13.2±12.0 months (
Table 2). Of the total 52 treatment periods, 18 (34.6%) were covered by insurance and 34 (65.4%) were not. Of the 34 discontinued treatment periods with known reason for withdrawal, 18 (52.9%) periods were discontinued after sufficient improvement, and 10 (29.4%) treatment periods were stopped by the patient. Five (14.7%) treatment periods were discontinued because of unsatisfactory responses or development of adverse events. One treatment period was discontinued because of the development of tongue cancer.
Table 2
Characteristics of all UST treatment period (n=52)
|
Characteristic |
Value |
|
Baseline PASI when starting UST treatment period*
|
12.9±5.8 |
|
Treatment duration (mo) |
13.2±12.0 |
|
Number of injections in each treatment period |
6.04±3.9 |
|
Cumulative dose in each treatment period (mg) |
271.8±173.6 |
|
PASI75†
|
31 (72.1) |
|
Time to PASI75 (wk) |
29.9±7.0 |
|
PASI90†
|
15 (34.9) |
|
Payment for ustekinumab treatment |
|
|
Covered by insurance |
18 (34.6) |
|
Patient's expense |
24 (46.2) |
|
Study participants |
10 (19.2) |
|
Reason for UST withdrawal‡
|
|
|
Making sufficient improvement |
18 (52.9) |
|
Unsatisfactory response or adverse event§
|
5 (14.7) |
|
Treatment discontinued at patient's request |
10 (29.4) |
|
Lost to follow-up |
1 (2.9) |

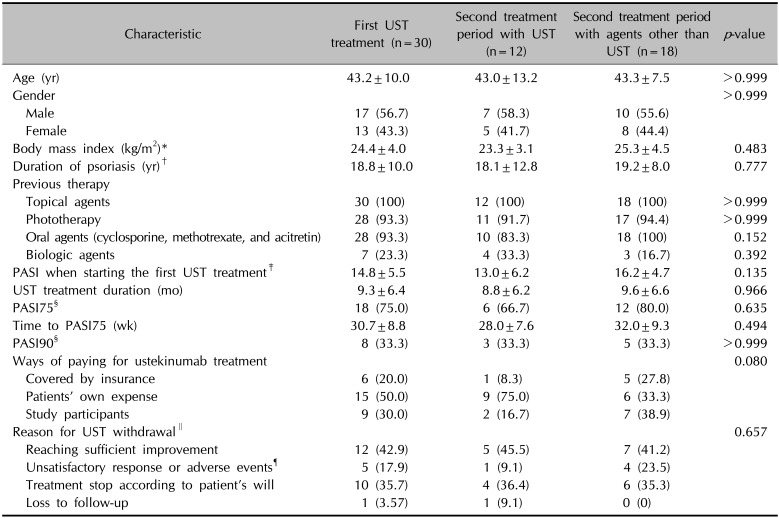
The outcome of the first ustekinumab treatment period and the choice of the next treatment modality
We analyzed the first ustekinumab treatment period in the 30 psoriasis patients because we speculated that the outcome of the first ustekinumab treatment period influenced the choice of subsequent treatment modalities (
Table 3). The baseline mean PASI score of the first ustekinumab treatment period was 14.8±5.48, which was higher than the baseline mean PASI of all 52 treatment periods (12.9±5.80).
Table 3
Characteristics of the first UST treatment period and a comparison of the patients who used UST as the second treatment and those who chose other treatments (n=30)
|
Characteristic |
First UST treatment (n=30) |
Second treatment period with UST (n=12) |
Second treatment period with agents other than UST (n=18) |
p-value |
|
Age (yr) |
43.2±10.0 |
43.0±13.2 |
43.3±7.5 |
>0.999 |
|
Gender |
|
|
|
>0.999 |
|
Male |
17 (56.7) |
7 (58.3) |
10 (55.6) |
|
Female |
13 (43.3) |
5 (41.7) |
8 (44.4) |
|
Body mass index (kg/m2)*
|
24.4±4.0 |
23.3±3.1 |
25.3±4.5 |
0.483 |
|
Duration of psoriasis (yr)†
|
18.8±10.0 |
18.1±12.8 |
19.2±8.0 |
0.777 |
|
Previous therapy |
|
|
|
|
|
Topical agents |
30 (100) |
12 (100) |
18 (100) |
>0.999 |
|
Phototherapy |
28 (93.3) |
11 (91.7) |
17 (94.4) |
>0.999 |
|
Oral agents (cyclosporine, methotrexate, and acitretin) |
28 (93.3) |
10 (83.3) |
18 (100) |
0.152 |
|
Biologic agents |
7 (23.3) |
4 (33.3) |
3 (16.7) |
0.392 |
|
PASI when starting the first UST treatment‡
|
14.8±5.5 |
13.0±6.2 |
16.2±4.7 |
0.135 |
|
UST treatment duration (mo) |
9.3±6.4 |
8.8±6.2 |
9.6±6.6 |
0.966 |
|
PASI75§
|
18 (75.0) |
6 (66.7) |
12 (80.0) |
0.635 |
|
Time to PASI75 (wk) |
30.7±8.8 |
28.0±7.6 |
32.0±9.3 |
0.494 |
|
PASI90§
|
8 (33.3) |
3 (33.3) |
5 (33.3) |
>0.999 |
|
Ways of paying for ustekinumab treatment |
|
|
|
0.080 |
|
Covered by insurance |
6 (20.0) |
1 (8.3) |
5 (27.8) |
|
Patients' own expense |
15 (50.0) |
9 (75.0) |
6 (33.3) |
|
Study participants |
9 (30.0) |
2 (16.7) |
7 (38.9) |
|
Reason for UST withdrawal∥
|
|
|
|
0.657 |
|
Reaching sufficient improvement |
12 (42.9) |
5 (45.5) |
7 (41.2) |
|
Unsatisfactory response or adverse events¶
|
5 (17.9) |
1 (9.1) |
4 (23.5) |
|
Treatment stop according to patient's will |
10 (35.7) |
4 (36.4) |
6 (35.3) |
|
Loss to follow-up |
1 (3.57) |
1 (9.1) |
0 (0) |

In the first ustekinumab treatment period, 6 (20.0%) treatment periods were covered by insurance, and 9 (30.0%) during the ustekinumab clinical trial. Compared to total 52 ustekinumab treatment periods, in the first ustekinumab treatment period, the proportion of treatment periods covered by insurance was low, while more patients used the ustekinumab during the clinical trial. During the first ustekinumab treatment periods, 12 (42.9%) were stopped after sufficient improvement, 10 (35.7%) at the patient's request, and 5 (17.9%) due to unsatisfactory response or development of adverse events. After a mean of 10.7±9.08 months, the patients started another treatment period and the mean PASI score at the start of the second treatment period was 10.6±5.10. Of the 30 patients, 12 (40.0%) selected ustekinumab again, while the remaining 18 (60.0%) selected other treatment modalities: six (20.0%) chose other biologics, five (16.7%) cyclosporine, three (10.0%) methotrexate, two (6.7%) phototherapy, one (3.3%) acitretin, and one (3.3%) a topical agent only.
To elucidate which factors influenced the choice of ustekinumab as the second treatment modality, we divided the enrolled patients into two groups: one that used ustekinumab again, and one that was treated with agents other than ustekinumab in the second treatment period (
Table 3). In the group that used ustekinumab again, 75% of the ustekinumab treatments were at the patients' own expense, whereas in the group treated with agents other than ustekinumab, 33.3% of the treatments were at the patients' own expense. In addition, the incidence of unsatisfactory response and adverse effects was 9.1% in the group who used ustekinumab in the second treatment, and 23.5% in the group using other agents. The analysis revealed no statistically significant differences in other demographic or treatment-related variables between the two groups.
Insurance coverage leads to choice of ustekinumab as a subsequent treatment
A total of 16 patients used ustekinumab for more than one treatment period. Of these, 12 patients underwent ustekinumab treatment periods twice, three patients three times, and one patient five times. Fifteen patients were receiving ustekinumab treatment at the end of the study. When comparing between 16 patients used ustekinumab for more than one treatment period and 14 patients who used ustekinumab once, the proportion of patients with previous experience of biologic agents was higher in the patients who used ustekinumab for more than one treatment period (23.3% of patients who used ustekinumab once and 31.3% of patients used ustekinumab for more than one treatment period;
p=0.031), while the proportion of patients with previous experience of oral agents was lower compared to 14 patients who used ustekinumab once (93.3% of patients who used ustekinumab once and 87.5% of patients used ustekinumab for more than one treatment period;
p=0.030). However, the analysis revealed no statistically significant differences in other demographic variables such as age, gender, body mass index, and duration of psoriasis (data not shown). To determine which factors led to choosing ustekinumab as a subsequent treatment, we compared the first and subsequent ustekinumab treatment periods (
Table 4). Between the first and subsequent ustekinumab treatment periods, the rate of reaching PASI75 and PASI90 and the time to reach PASI75 were similar. The analysis on the ways of paying for ustekinumab treatment showed that 20% of patients were covered by insurance in the first ustekinumab treatment period, but 54.5% in the subsequent ustekinumab treatment period. A further analysis on 16 patients who used ustekinumab more than one periods showed a statistically significant difference in the ways of paying for ustekinumab treatment between the first and second treatment period: the proportions of the ways of paying for first ustekinumab treatment were 6.3%, 68.8%, and 25.0% for covered by insurance, patient's expense, and study participants, while for second ustekinumab treatment, 62.5%, 31.3%, and 6.3%, respectively (
p=0.011). In addition, there was a significant difference in the duration of ustekinumab treatment period between the first and second treatment period. The duration of first ustekinumab treatment period was 8.9±5.5, while that of second ustekinumab treatment period was 23.9±16.1 (
p=0.004).
Table 4
Comparison between the first and subsequent UST treatment periods

|
First UST period (n=30) |
Subsequent UST treatment periods (n=22) |
|
PASI when starting each treatment period*
|
14.8±5.5 |
11.0±5.7 |
|
UST treatment duration of each period (mo) |
9.3±6.4 |
19.5±15.8 |
|
PASI75†
|
18 (75.0) |
14 (73.7) |
|
Time to PASI75 (wk) |
30.67±8.8 |
27.1±5.7 |
|
PASI90†
|
8 (33.3) |
7 (36.8) |
|
Ways of paying for ustekinumab treatment |
|
|
|
Covered by insurance |
6 (20.0) |
12 (54.5) |
|
Patients' own expense |
15 (50.0) |
9 (40.9) |
|
Study participants |
9 (30.0) |
1 (4.5) |

Go to :

DISCUSSION
Recent advances in immunologic research have increased understanding about the pathophysiology of psoriasis, and new biologic agents have been introduced for psoriasis treatment. Unlike conventional systemic agents or phototherapy, the new biologic agents can be used continuously and this was reported to result in greater and sustained therapeutic effects
3. In the case of ustekinumab, it was reported that continuous treatment is the most effective option for moderate-to-severe psoriasis
34. Continuous treatment with ustekinumab for 3 years maintained PASI75 in more than 80% of patients, whereas discontinuation of ustekinumab at 16 weeks resulted in failure to maintain PASI75 in 50% of patients
34. Furthermore, the lack of cumulative toxicity after the long-term treatment supports the continuous use of biologic agents in the treatment of psoriasis
3411. However, some patients are known to discontinue biologic agents because of the relative expense of the agents, surgery, pregnancy, lactation, and low compliance with the treatment
12.
In this study, we enrolled a total of 30 patients who started the ustekinumab treatment but discontinued the treatment and were followed up for the treatment of psoriasis. We analyzed the treatment behaviors of these patients to reveal the factors affecting ustekinumab treatment in real clinical practice. A total of 52 ustekinumab treatment periods were documented in these 30 patients. The mean duration of an ustekinumab treatment period was 13.8±12.1 months, and 72.1% of treatment periods reached PASI75 and 34.9% reached PASI90. In this study, the effectiveness of intermittent ustekinumab treatment was slightly lower than the previously reported efficacy of continuous ustekinumab treatment
59. However, this result is comparable or superior to that of conventional systemic treatment modalities such as cyclosporine, methotrexate, and acitretin
313. Because intermittent use of ustekinumab showed comparable or superior effectiveness to previous systemic agents, we concluded that discontinuing ustekinumab was not due to lack of effectiveness. The analysis of the reasons for ustekinumab discontinuation confirmed our conclusion, with more than 80% of treatment periods discontinued when sufficient improvement was reached or at the patient's request. In contrast, only 14.7% discontinued the treatment period due to unsatisfactory response or adverse events. These results suggest that the intermittent treatment with ustekinumab is not due to lack of effectiveness or adverse events, but the external factors other than the effectiveness of ustekinumab.
To determine the external factors causing discontinuation of ustekinumab treatment, we performed subgroup analysis comparing between first and subsequent ustekinumab treatment. Hypothesizing that the outcomes of the first ustekinumab treatment period determined the subsequent intention of using ustekinumab, we analyzed the first ustekinumab treatment period by comparing the patients who continued to use ustekinumab for a second period, and the patients who went on to use agents other than ustekinumab. In the analysis, we found that the proportion of treatment at their own expense was higher in the group of patients who used ustekinumab for a second treatment period, but the difference was not significant. In addition, the comparison between the first and second ustekinumab treatment periods found that the proportion of ustekinumab treatment covered by insurance in the second ustekinumab period was higher compared to that of the first ustekinumab treatment period and the duration of ustekinumab treatment was longer in the second ustekinumab treatment period. We concluded that the method of payment for ustekinumab treatment plays an important role in determining the subsequent treatment modalities and the choice of ustekinumab for subsequent treatments.
Compared to conventional systemic agents for psoriasis, newly introduced biologic agents are more expensive
314. Particularly in South Korea, patients with psoriasis must meet strict criteria in order to have ustekinumab treatment covered by insurance. If these conditions are not met, the patient has to undergo ustekinumab treatment at their own expense. For this reason, 65.3% of the 30 patients who underwent intermittently ustekinumab treatment were uninsured. Moreover, 75% of those who chose ustekinumab again after the first treatment underwent ustekinumab treatment at their own expense. The patients who underwent intermittent and repetitive ustekinumab treatment were satisfied with the outcome of ustekinumab treatment, but were uninsured and could not afford the economic burden of continuous treatment. This was confirmed by the findings that the effectiveness of intermittent ustekinumab treatment was comparable or superior to that of conventional systemic treatment modalities and the proportion of patients who experienced the biologic agents was higher in the patients who used ustekinumab repetitively. Moreover, it was also confirmed by that the proportion of ustekinumab treatment periods covered by insurance was significantly higher in second ustekinumab treatment periods and the duration of treatment was longer: the insurance coverage for the ustekinumab treatment resulted in using ustekinumab more actively and continuously.
Lastly, the result of this study can provide guidance for the ustekinumab treatment for psoriasis. In real clinical practice, some psoriatic patients who responded well to ustekinumab discontinue their treatment. However, there was no guidance on whether it is appropriate to resume the ustekinumab treatment in these patients. The result that the effectiveness of ustekinumab was maintained in the subsequent treatment suggests the resuming the use of ustekinumab in the patients.
This study has some limitations. Some treatments of enrolled patients were not covered by the insurance and they had to pay for ustekinumab treatment by themselves. The differences in the characteristics of the enrolled patients may have led to difference between the results of this study and those of previous clinical trials. However, considering that the insurance coverage for ustekinumab does not apply in all cases in real clinical practice, the results of this study can be a reflection of practical aspects of ustekinumab treatment.
In conclusion, we found that the patients who underwent intermittent and repetitive ustekinumab treatment discontinued their ustekinumab treatment due to the economic burden, but they chose another ustekinumab treatment intentionally. Thus, the economic burden can be the major determining factor resulting in short-term intermittent and repetitive ustekinumab treatment for moderate-to-severe psoriasis. However, in the case of psoriasis treatment with biologic agents, long-term continuous treatments have been reported to be effective and safe compared to intermittent, “as needed” treatment
1516. Recently, the changes in the regulation for the insurance for biologic agents have reduced the burden of ustekinumab treatment in Korea, though, it is also necessary to alleviate the requirements for insurance coverage so that more psoriasis patients can use the newly introduced biologic agents. The expansion of insurance coverage for biologic agents can increase effectiveness and safety in the management of psoriasis.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download