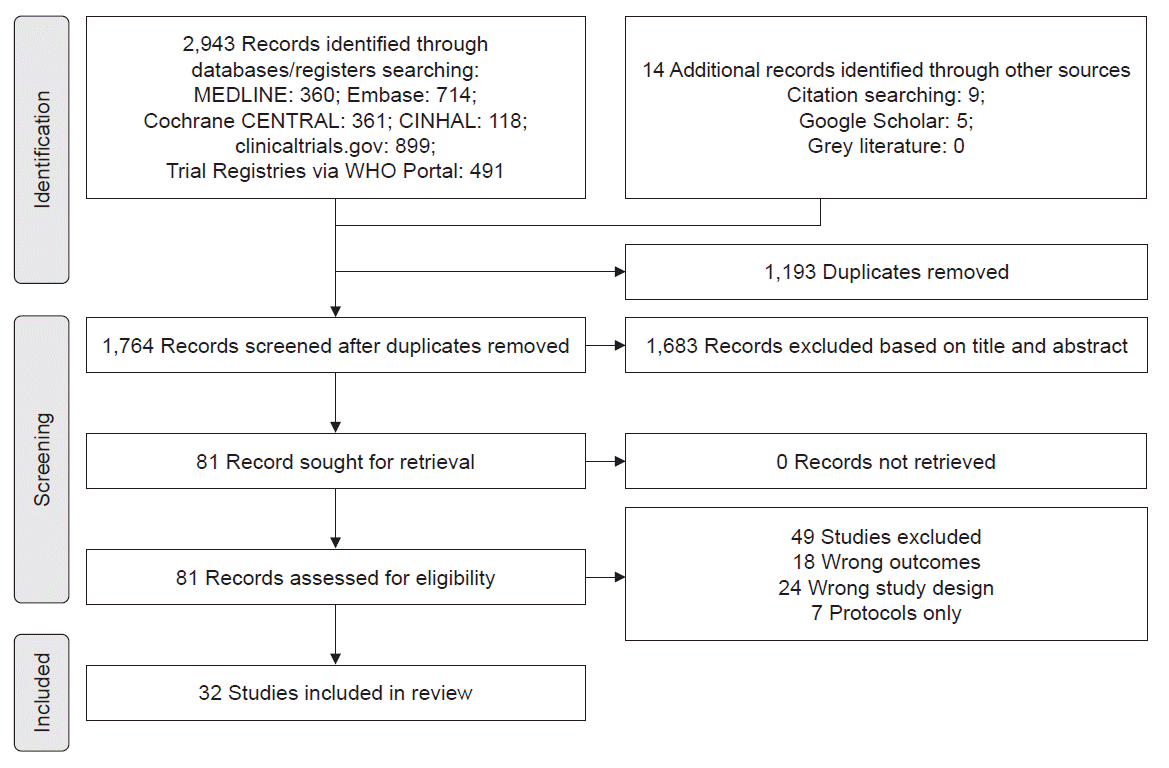
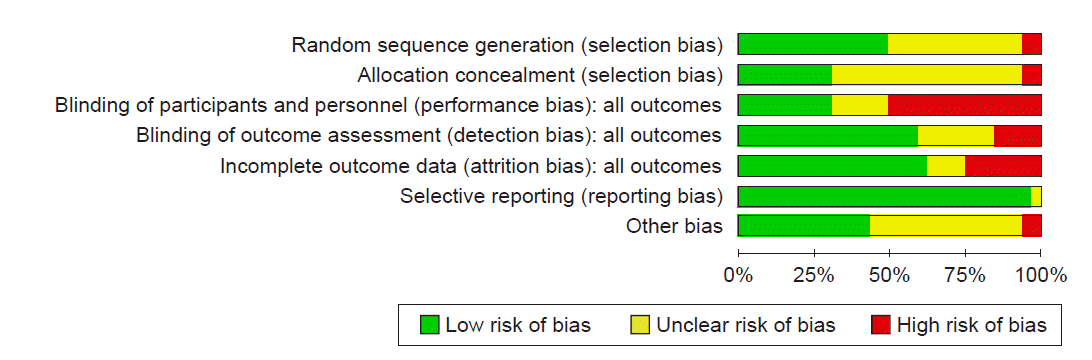
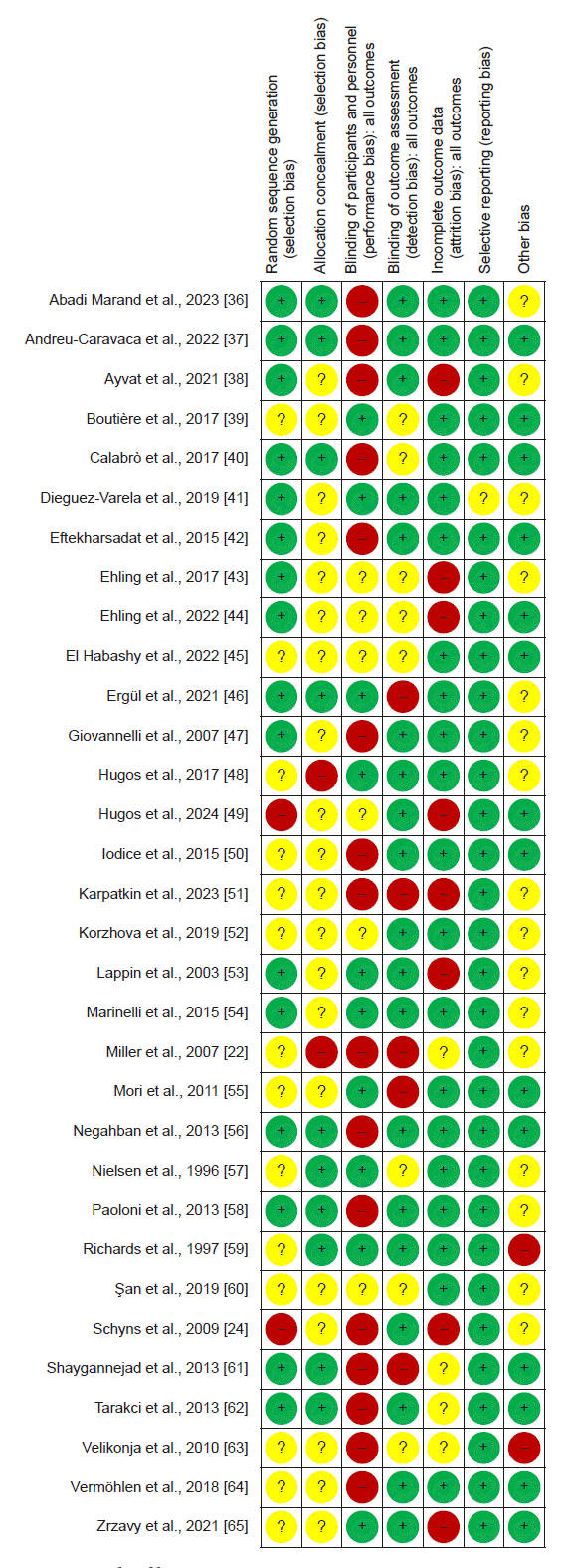
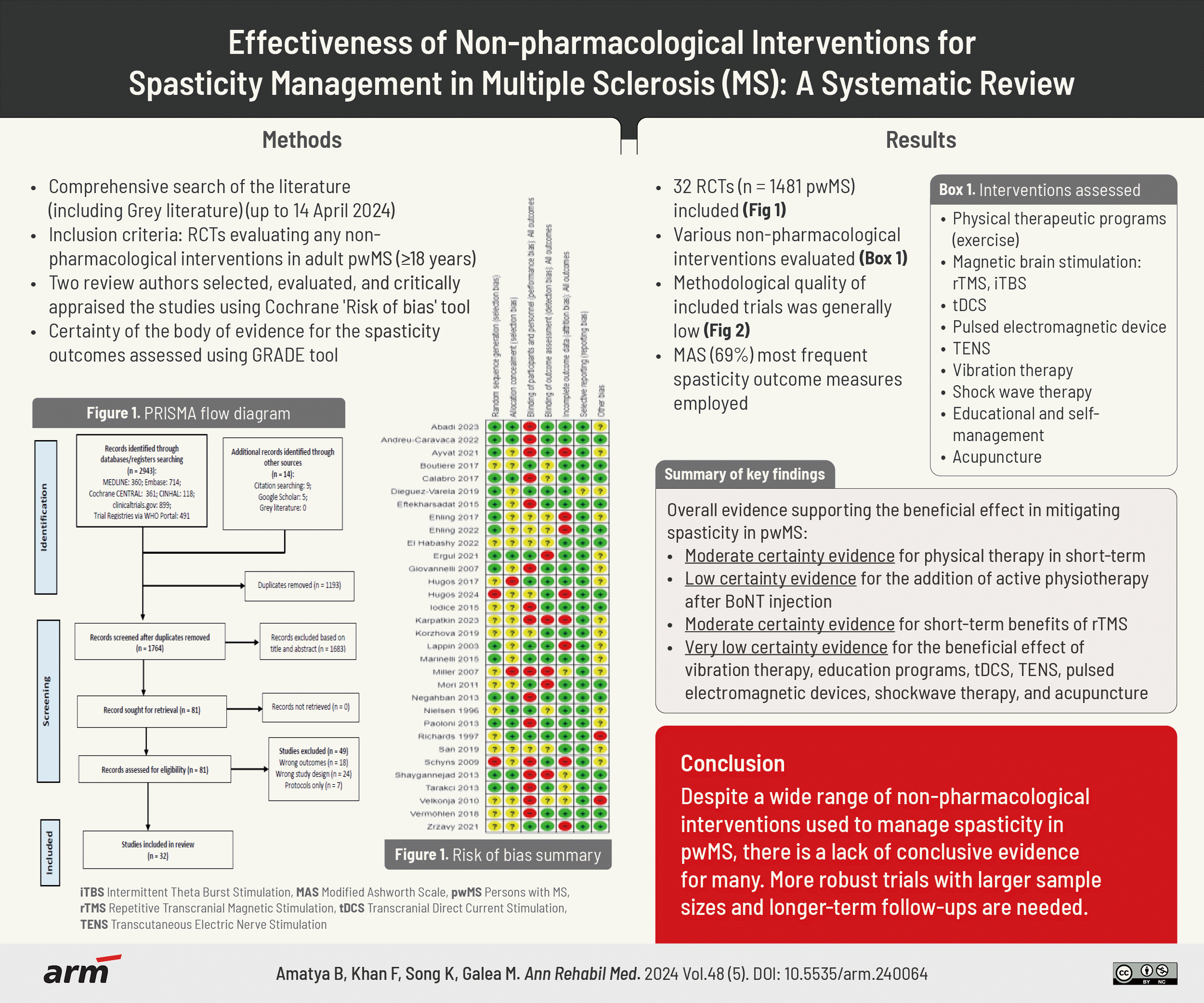
Abstract
Notes
CONFLICTS OF INTEREST
The review authors are professionals in the field of Physical and Rehabilitation Medicine who wish to provide the best possible service to their patients. No potential conflict of interest relevant to this article was reported.
FUNDING INFORMATION
This review was supported by the internal resources of the Department of Rehabilitation Medicine, Royal Melbourne Hospital, Australia. No external support was received. Furthermore, no commercial party has a direct financial interest in the results reported in this article, nor will any commercial party confer a benefit upon the authors or any organization with which the authors are associated.
AUTHOR CONTRIBUTION
Conceptualization: Amatya B, Khan F. Methodology: Amatya B, Khan F. Formal analysis: Amatya B, Song K. Funding acquisition: Khan F. Project administration: Amatya B, Khan F. Visualization: Amatya B, Khan F, Song K, Galea M. Writing – original draft: Amatya B. Writing – review and editing: Amatya B, Khan F, Song K, Galea M. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary Material S1.
REFERENCES
Table 1.
| Reference | Participants’ characteristic | Intervention | Outcome measure, assessment time point | Result | Certainty of evidence | |
|---|---|---|---|---|---|---|
| Spasticity outcome | Other outcome | (GRADE) | ||||
| Physical therapeutic (exercise) program | ||||||
| Abadi Marand et al., 2023 [36], Iran | N=64: TG=32, CG=32 | TG: DNS exercises | Spasticity: MSSS-88, MAS | · Significant improvement in spasticity MSSS-88 at post-intervention & 17 weeks follow-up compared with the CS group, (group×time) (F=11.28, p>0.001) | At post-intervention & 17 weeks significant improvement in DNS group compared with the CS group (group×time): | ⊕⊕⊝⊝ Low |
| TG: age=40.4±6.0 yr, M/F: 17/15, SPMS=21, RRMS= 11, EDSS: 4.1±1.1, DD: 14.4±5.2 yr | CG: CS | Balance: BBS, postural stability | · No effect on MAS scores (p>0.05) | · BBS (F=65.8, p<0.001) | ||
| CG: age= 40.7±6.2 yr, M/F: 18/14, RRMS=10, SPMS=22, EDSS: 3.8±1.0, DD: 12.8±5.9 yr | Frequency: 15 60-min sessions 3 times per week for 5 weeks | Falls: falling rate; | · Trunk Impairment Scale (F=40.6, p<0.001) | |||
| Fear of falling: activities-specific balance confidence, Biodex Balance System | · Postural stability (F=16.9, p<0.001) | |||||
| Trunk function: Trunk Impairment Scale | · Activities-specific balance confidence (F=10.1, p>0.001) | |||||
| Mobility: MSWS-12, TUG | · Reduced falling rate (F=9.0, p<0.001) | |||||
| Baseline, post intervention (5 wk) & 17 wk | · TUG (F=9.4, p<0.001) | |||||
| · MSWS-12 (F=3.8, p<0.05) | ||||||
| · No AEs from both interventions | ||||||
| Andreu-Caravaca et al., 2022 [37], Spain | N=30: TG=18, CG=12 | TG: strength training (fast-velocity concentric) | Spasticity: pendulum test | · Post-intervention significant improvement in spasticity, with differences between groups, in first swing excursion (right leg: p<0.01, ES=-1.4; left leg: p<0.05, ES=-1.2), number of oscillations (right leg: p=0.001, ES=-0.4; left leg: p<0.05, ES=-0.4) & duration of oscillations (left leg: p<0.01, ES=-0.6) | · Significant improvement with differences between groups in muscle activity (p<0.05, ES=-0.8) & maximal neural drive (p<0.05, ES=-0.8) | ⊕⊕⊝⊝ Low |
| Age: 46.0±10.4 yr, M/F=15:15, RRMS=27, SPMS=3, EDSS: 3.2±1.5 | CG: usual care | Muscle activity: vastus lateralis (sEMG, peak sEMG), voluntary activation (central activation ratio), muscle contractile function | · Voluntary muscle activation (central activation ratio) increased after intervention in IG (p<0.05, ES=-0.4) | |||
| Frequency: 3 sessions/week on alternating days for 10 weeks | Pre & post intervention (10 wk) | · Contractile properties remain unchanged in both groups | ||||
| · No AEs | ||||||
| Calabrò et al., 2017 [39], Italy | N=40 (RRMS): TG=20, CG=20 | TG: RAGT with VR | Spasticity: MAS | · No significant effect on spasticity between group (p>0.05, ES=-0.01, 95% CI=-0.5 to 0.5) or within group (p>0.05 for both groups) | · Non-significant difference between the groups for BBS (ES=-0.02, 95% CI=-2.4 to 2.4, p>0.05) & TUG (ES=-0.06, 95% CI=-0.4 to 0.5, p>0.05) | ⊕⊕⊝⊝ Low |
| TG: age=44 (40–48) yr, M/F=7/13, EDSS: 4.4 (4–4.9), DD=11.5 (8–14) yr | CG: RAGT only | Function & balance: TUG, BBS, FIM | · Significant moderate-to-large effect for positive attitude (ES=-0.5, 95% CI=-3.6 to 2.6) & problem-solving (ES=-0.9, 95% CI=-2.1 to 0.3, p<0.01) | |||
| CG: age=41 (38–47) yr, M/F=8/12, EDSS: 4.75 (4.1–5.5), DD=11.5 (8–16) yr | Frequency: 5 sessions (30 min general conditioning+40 min RAGT) per week for 8 weeks | Cognition: COPE, HRSD | · No AEs | |||
| Pre & post intervention (8 wk) | ||||||
| Eftekharsadat et al., 2015 [42], Iran | N=30: TG=15, CG=15 | TG: postural stability training program Biodex Balance System with VR | Spasticity: MAS | · No significant difference between groups on spasticity (MAS) scores of the knee & hip (p>0.05) | · Significant improvement in functioning but not in balance | ⊕⊕⊝⊝ Low |
| TG: age=33.4±8.1 yr, M/F=5/10, DD=5.8+3.9 yr | CG: no intervention | Function & balance: MMT, TUG, Romberg test, BBS | · No significant difference between groups on MMT scores of the wrist, hip, & knee, or BBS scores (p>0.05 for all) | |||
| CG: age=37.0±8.3 yr, M/F=3/12, DD=8.3+4.3 yr | Frequency: 2 sessions | Fall risk & postural stability tests: FRI, and OSI | · Significant improvement in TUG scores in intervention group (p=0.01) | |||
| (20 min) per week for 12 weeks | Pre & post intervention | · Significant improvement in FRI (p<0.001) & OSI (p<0.01) in intervention group | ||||
| · No report on AEs | ||||||
| Ergül et al., 2021 [46], Iran | N=26: TG=13, CG=13 | TG: SSE of hamstrings, quadriceps, hip adductors, plantar flexors muscles | Spasticity: MAS | Significant reduction in spasticity in both groups (p<0.05), but no difference between groups | · Compared to baseline both groups showed significant improvement of functional tests, decrease of pain, increase of ROM & increase of HRQOL (p<0.05) | ⊕⊕⊝⊝ Low |
| TG: age=45.3±12.0 yr, M/F=7/5, DD=13.4±7.9 yr | CG: FSE | Function: TUG, T25FWT, active ROM assessment | In SSE group: | · No significant differences between both groups in all variables before & after treatment (p>0.05) | ||
| CG: age=43.8±7.6 yr, M/F=5/7, DD=13.4±7.9 yr | Frequency: 3 sessions (25–30 min) per week for 4 weeks | Pain: VAS | · Strong correlation between decreased spasticity of quadriceps & improved function (TUG, r=0.7, p<0.01) | · No report on AEs | ||
| QOL: EQ-5D-5L | · Strong correlation between decreased spasticity of quadriceps & improved walking (T25FWT, r=0.7, p<0.01) | |||||
| Pre & post intervention (4 wk) | · Moderate correlation between decreased spasticity of hip adductors & improved strength (TUG, r=0.7, p<0.05) | |||||
| In the FSE group | ||||||
| · Moderate correlations between decreased spasticity & increased ROM (r=0.7, p<0.05) & between increased ROM & functional improvement (p<0.05) | ||||||
| · Strong correlation between decreased spasticity & increased HRQOL (p<0.01) | ||||||
| Giovannelli et al., 2007 [47], Italy | N=37: TG=20, CG=20 (18 at follow-up) | BoNT & PT (passive or active exercise & a stretching regimen) | Spasticity: MAS & VAS | Significant decrease in MAS score in TG from baseline at (MD±SD): | · No adverse events post BoNT injections | ⊕⊕⊝⊝ |
| TG: age=46.0±9.0 yr, M/F=2/18, EDSS: 5.8±1.3 | Frequency: 40 min session daily/15 days after BoNT | Function: EDSS | · Week 2: TG=-0.91±0.52, CG=-0.39±0.50, p<0.01 | Low | ||
| CG: age=48.1±7.5 yr, M/F=2/16, EDSS: 6.0±1.1 | · Week 4: TG=-1.0±0.69, CG=-0.28±0.46, p<0.01 | |||||
| Control group: BoNT alone | Baseline, & weeks 2, 4, & 12 | · Week 12: TG=-0.95±0.78, CG=-0.28±0.46, p<0.01 | ||||
| Mean (%) difference in MAS between baseline & 12 weeks: TG=-0.95 (26.1), CG=-0.28 (7.7) (p<0.01) | ||||||
| Significant improvement in spasticity in VAS rating scale (MD± SD): | ||||||
| · Week 2 to week 4: TG=1.77± (0.87), CG=0 (1.08), p<0.01 | ||||||
| · Week 4 to week 12: TG=2.68 (1.08), CG=1.06 (1.16), p<0.01 | ||||||
| Hugos et al., 2024 [49], USA | N=231: IG=115, CG=116 | IG: STC: stretching exercise & education program | Spasticity: MSSS-88, NRS | · No significant difference in MSSS scores between STC and ROM at 1 month (MD=0.28, 95% CI=-9.45 to 10.01, p>0.05) or 6-month (MD=-0.86, 95% CI=-12.2 to 10.5) | · Significant difference between groups in fatigue (MFIS, NRS) and impact of MS (MSIS) | ⊕⊕⊝⊝ Low |
| IG : age=53.7±12.1 yr, M/F=16/99, RRMS=64, PPMS=50, DD= 15.3 yr | CG: ROM exercises & education program | Function: MSWS, TUG, T25MW | · Significant improvements in group mean MSSS scores at 1 and 6 months in both groups | · No significant improvement in function (MSWS, TUG, T25FW) | ||
| CG: age=55.1±11.1 yr, M/F=16/100, RRMS=54, PPMS=62, DD=17.0 yr | Frequency: 2 h/wk classes; exercises: 15–30 mins/day | Fatigue: MFIS | ||||
| Others: PSQI, PROMIS (short form 8a), MSIS | ||||||
| At 1 and 6 months | ||||||
| Negahban et al., 2013 [56], Iran | N=48 (12 in each TGs & CG) | 4 parallel groups: TG I: Swedish massage; TG II: exercises (strength, stretch, endurance & balance); TG III: combined massage & exercise | Spasticity: MAS (ankle plantar flexors) | · Significant improvement in MAS in TG I: MD±SD=0.54±0.6, p<0.01) & TG II: 0.47±0.7, p<0.05), but not in TG III: 014±0.8, p>0.05) | · FSS scores improved in all TGs but worsened in CG | ⊕⊕⊕⊝ Moderate |
| TG I: age=36.3±7.6 yr, M/F: 2/10, EDSS=3.8±1.4, DD=48.7±97.1mts | CG: standard medical care | Pain: VAS | · Significant worsening in MAS scores in CG: -0.33±0.5, p<0.05 | · No significant difference in MSQL-54 between groups | ||
| TG II: age=6.7±6.7 yr, M/F: 2/10, EDSS: 3.5±1.1, DD=102±81.1 mts | Frequency: three 30 min sessions a week for 5 weeks | Fatigue: FSS | · No significant difference between groups | · Significant improvement in TUG in TG I (4.78±5.9, p<0.01 | ||
| TG III: age=36.7±7.6 yr, M/F: 2/10, EDSS: 3.6±1.4, DD=115.3±78.3 mts | Function & gait/balance: BBS, TUG, 10MWT | · TG I showed significantly larger change scores in all outcome measurements than the CG | ||||
| CG: age=36.8±8.7 yr, M/F: 2/10, EDSS=3.8±1.4, DD=86.6±34.3 mts | HRQOL: MSQLI-54 | · TG II showed significant improvement in all outcomes except pain VAS scores than CG | ||||
| Pre & post intervention (5 wk) | · No intolerance or the AEs of intervention | |||||
| Tarakci et al., 2013 [62], Turkey | N=110: TG=55, CG=55 | Group exercise training including strength training, balance & coordination, core stabilisation, etc. | Spasticity: MAS | Significant improvements in all lower limb spasticity (MAS scores) (p<0.05 for all) post-treatment: | Significant improvements in TG compared to CG: | ⊕⊕⊕⊝ Moderate |
| TG: age=41.49±9.37 yr, M/F=17/34, EDSS: 4.38±1.37, DD: 9±4.71 yr | Frequency: 3 sessions (1 h) a week for 12 weeks | Function & balance: BBS, 10MWT, 10-steps climbing test | · R hip flexors: p<0.001, ES=1.01 | · Balance: BBS score increased 4.33 in the exercise group, while a decrease of 2.33 in CG (p<0.01) | ||
| CG: age=39.65±11.18, M/F=18/30, EDSS: 4.21±1.44, DD: 8.42±5.38 yr | Fatigue: FSS | · L hip flexors: p=0.015, ES=0.3 | · Walking: 10MWT-decreased 2.72 seconds in TG, while increased by 1.44 in CG (p<0.001) | |||
| QOL: MSIQOL | · R hamstring: p<0.001, ES= 0.92 | · Reduction in fatigue: FSS score (<0.001) | ||||
| Pre & post intervention | · L hamstring: p<0.001, ES=0.8 | · Improve QOL: MusiQOL (p<0.05) | ||||
| · R Achilles: p<0.05, ES=0.54 | · No AEs | |||||
| · L Achilles: p<0.001, ES=0.95 | ||||||
| Velikonja et al., 2010 [63], Slovenia | N=20: number of participants in each group not stated | TG: sports climbing (climbing wall, climbing belt and top rope system, climbing up and down wall) CG: yoga (stretching, strengthening exercises, breathing exercises, isometric muscle contraction & relaxation) | Spasticity: MAS | · No significant improvements in spasticity after both interventions (p>0.05) | · Significant reduction in fatigue in TG (32.5%, p<0.05), while CG had no effect | ⊕⊝⊝⊝ Very low |
| TG: (median): | Frequency: once a week for 10 weeks | Function: EDSS | · SC group had 25% reduction (p=0.046) in EDSS per year; before 4.0 (3.0–4.0) after 3.0 (2.5–4.0) | · Improved fatigue impact in TG before & after: MFIS 40.0 (36.5–53.0) to 27.0 (21.5–45.5), p<0.05 and MFIS cognition 17.0 (8.5–21.5) to 8.0 (6.0–19.5), p<0.05; MFIS physical 25.0 (21.5–28.5) to 19.0 (9.0–26.5), p<0.05 | ||
| age=42, EDSS: 4.0 | Cognition: (executive function, attention span), Mazes subtest of Executive module from NAB, TOL, Brickenkamp d2 test | · No differences in executive function and mood | ||||
| CG (median): | Mood: CES-D | · Increase in selective attention performance in the yoga group (17% increase; baseline: 151.0 (94.5–175.5); after: 176.5 (116.5–191.3; p<0.01) | ||||
| age=41, EDSS: 4.2 | Fatigue: MFIS | · No report on AEs | ||||
| Baseline & after treatment (10 wk) | ||||||
| Vermöhlen et al., 2018 [64], Germany | N=70: TG=32, CG=38 | TG: hippotherapy & standard care | Spasticity: NRS | · Spasticity significantly improved in TG (NRS) from baseline to week 12: change from baseline=-1.7 points, no change in CG (-0.6), MD: -0.9 (95% CI: -1.9 to -0.1, p<0.05) | · Significant improvement in balance (BBS score, MD=2.33, 95% CI=0.03 to 4.63, p=0.047) | ⊕⊝⊝⊝ Very low |
| TG: age (Md, IQR)=50 (45–53), M/F=3/27; EDSS<5.0 in 33%, DD (Md, IQR): 16.5 (11–20) | CG: standard care | Balance: BBS | · Benefit was largest for the subgroup with an EDSS≥5 (BBS score SD=5.1, p=0.001) | |||
| CG: age=51 (47–56), M/F=10/27, EDSS<5.0 in 30%, DD: 17.6 (11–27) | Frequency: once a week for 12 weeks | Others: FSS, MSQOL-54, VAS pain scale | · Significant improvement in fatigue (FSS score, MD=-6.8, p<0.05) | |||
| Baseline, 6 weeks & 12 weeks | · Significant improvement in QOL (MSQol-54, physical (MD=12, p<0.001) & mental health (MD=14.4, p<0.001) | |||||
| · 49 AEs (IG: 22 AEs in 13 patients, CG: 27 AEs in 15 patients); 3 serious AEs due to the necessary hospitalization ( IG: 1 SAE [MS relapse], CG: 2 SAEs [MS relapse & infection]) | ||||||
| Zrzavy et al., 2021 [65], Austria | N=39: TG I=13, TG II=8, CG=11 | TG I: rehabilitation program+hypoxic endurance training | Spasticity: MSSS-88 | · Significant lower spasticity scores (MSSS-88) in both endurance training groups at 14 days (TG I: p=0.012; TG II: p<0.05) | · Significant improvement in walking endurance (6MWT) in TG I only at 14 days (p=0.001) | ⊕⊕⊝⊝ Low |
| TG II: rehabilitation program+normoxic endurance training | Fatigue: Erschöpfungsinventar bei Multipler Sklerose (WEIMuS), MFIS | · Significant improvement in spasticity at 1 week in TG II (p<0.01) | · Fatigue scores improved significantly in all groups, but these improvements reached faster in TG I (p<0.01) & TG II (p=0.004) | |||
| TG I: age=43.9±6.2 yr, M/F=4/13, EDSS (MD, range): 4 (4–5), DD: 13±9 yr | CG: rehabilitation program | Walking: 6MWT | · No improvement in CG | · No report on AEs | ||
| TG II: age=41.5±11.3 yr, M/F=4/7, EDSS=3.5 (2–5), DD=6±5 yr | Frequency (group 2 & 3): 45 minutes over 12 days (separated by a day without training) | Depressive symptoms: ADS | ||||
| CG: age=43.9±6.1 yr, M/F=2/9, EDSS=6 (4–5), DD=14±7 yr | Baseline, 7 days & 14 days | |||||
| Magnetic brain stimulation | ||||||
| Boutière et al., 2017 [39], France | N=17: TG= 9, CG=8 | TG: iTBS (10 bursts of 3 stimuli (50 Hz) repeated at theta frequency (5 Hz) every 10 seconds for a total of 600 stimuli (192 s) adjuncts to rehabilitation program | Spasticity: MAS, VAS | · Significant improvement in VAS spasticity score in iTBS group (p=0.026) | · Significant effect of iTBS on the balance of the connectivity degree between the stimulated & the homologous primary motor cortex (p<0.01) | ⊕⊕⊕⊝ Very low |
| TG: age=48.2±9.4 yr, M/F=5/4, SPMS=6, RRMS=3, EDSS (Md, IQR): 6 (4–7), MAS: 7.6±4.7, DD: 12.2±8.2 yr | CG=sham iTBS | Brain function: resting-state fMRI | · MAS score improved in both groups, but no significant difference between groups (p>0.05) | · No effect of iTBS on global topology of brain network, suggesting that iTBS over the primary motor cortex does not alter global organization of brain network | ||
| CG: age=55.4±11.1 yr; M/F=4/4, SPMS=7, RRMS=1, EDSS=6 (6–6.5), MAS=5.6±2.7, DD=18.7±11 yr | Frequency: once a day for 13 consecutive working days | Baseline, day after the last session of iTBS (week 3) & at the end of the 5-week rehabilitation | · Changes in inter-hemispheric balance were correlated with improvement of spasticity (p<0.05) | · No report on AEs | ||
| program (week 5) | ||||||
| Dieguez-Varela et al., 2019 [41], Spain | N=17: TG=10, CG=7 | TG: iTBS (10 bursts with 3 pulses at 50 Hz repeated at 200 ms intervals (5 Hz) every 10 s for a total of 600 stimuli | Spasticity: MAS, H/M amplitude ratio, PCS in the soleus muscle | · No significant differences between the two groups in MAS & other clinical variables (PCS, adductor tone, joint balance, foot support & the Hauser ambulatory index) at any of the assessment time points | · AEs: 2 reported (subjective weakness in the right foot after the second session & a subsequent fall; & mild headache) | ⊕⊕⊝⊝ Low |
| Age: 49.8±9.8 yr, M/F: 7/10, DD=12.4±6.4 yr | CG: sham stimulation | Assessments: immediately after 1 (S1), 5 (S5) & 10 (S10) sessions; 1 week (day 19) & 2 weeks after treatment | · Significant decrease in H/M amplitude ratio from baseline (0.42±0.29) vs. S1 (0.35±0.25, p<0.05), vs. S5 (0.35±0.26, p<0.01) & vs. S10 (0.35±0 .27, p<0.01) | |||
| Frequency: 10 daily sessions for 2 weeks | · Effect was maintained up to 1 week after the last stimulation session: S1 (0.42±0.29) vs. day 19 (0.36±0.28), p<0.05 | |||||
| · No significant changes in CG | ||||||
| Korzhova et al., 2019 [52], Russia | N=34 SPMS: TG I: 12, TG II: 12, CG: 10 | TG I: rTMS (20 Hz) & PT 45–55 min spastic muscle strengthening | Spasticity: MAS, SESS, NAS | · At T1, significant reduction MAS in both TG I (MD= -1.0, 95% CI: -1.3, -0.6, p<0.001), & TG II (MD=-1.5, 95% CI: -2.1, -0.8, p<0.001), but not in CG (MD= -0.2, p<0.05) | · Significant reduction in pain in TG I only: MD (95% CI) =-5.0 (-8.6, -1.4) but not in TG II or CG | ⊕⊕⊕⊝ Moderate |
| TG I: age=38 (29–54) yr, M/F=4/8, EDSS=6.5 (6–6.5) | sessions | Fatigue: MFIS | · At T1 significant reduction of | · Significant reduction in MFIS score in TG I only: -7.0 (-11.7, -2.3), but not in TG II or CG | ||
| TG II: age=47 (43–53) yr, M/F=5/7, EDSS=6.5 (6–6.5) | TG II: iTBS | Other: pain level scale | spasticity level as measured by SESS in both TG I: MD (95% CI)=-1.0 (-2.0, 0.0) & TG II: MD=-1.0 (-1.5, -0.5), but not in CG: MD=-0.5 (-1.3, 0.3) | · No AEs | ||
| CG: age=45.0 (41–47) yr, M/F=5/5, EDSS=6.5 (6–6.5) | CG: sham rTMS | Baseline (T0), post intervention (T1, 10 sessions), 2 weeks (T2) & 12 weeks (T3) | · At T1 significant reduction in NAS scores TG I: MD (95% CI=-2.8 (-4.0, -1.5), TG II: MD=1.6 (-2.9, -0.2), & CG=-1.3 (-2.3, -0.3) | |||
| Frequency: 1 stimulation per day for 5 sessions for 2 weeks | · At T3 significant reduction in SESS score only in iTBS group | |||||
| Mori et al., 2011 [55], Italy | N=30: TG I: 10, TG II: 10, CG: 10 | TG I: iTBS (10 bursts, with each burst of 50 Hz 3 stimuli, repeated at a theta frequency of 5 Hz every 10 seconds, for a total of 600 stimuli (200 s) & exercise therapy | Spasticity: MAS, MSSS-88 | Significant improvement in TG I: | Significant improvement in TG I on: | ⊕⊕⊝⊝ Low |
| TG I: age=39.1±10.7 yr, M/F=7/3, EDSS=3.6±1.2 | TG II: iTBS alone | Fatigue: FSS | · MAS from the stimulated leg (2.1±0.4 before treatment; 1.3±0.4 after treatment; p<0.05) | · FSS (39.5±4.2 before treatment; 31.6±4.6 after treatment; p<0.05) | ||
| TG II: age 38.3±11.9 yr, M/F=5/5, EDSS=3.5±1.0 | CG: sham stimulation & exercise therapy | ADLs: BI | · MSSS-88 (74.3±11.4 before treatment; 53.2±10.9 after treatment; p<0.001) | · BI (92.5±2.4 before treatment; 95.0±1.85 after treatment; p<0.05) | ||
| CG: age=37.7±12.3 yr, M/F=6/4, EDSS=3.8±1.6 | Frequency: 10 bursts, 2 weeks | HRQOL: MSQOL-54 | · In TG II, significant improvement in MAS (3.3±0.8 before treatment; 1.6±0.8 after treatment; p<0.05) | · MSQOL-54 physical health composite (59.7±2.7 before treatment; 64.8±2.7 after treatment; p<0.05) scores after treatment | ||
| Baseline & after treatment (2 wk) | · No significant changes in CG | · None of the measured scales showed significant changes in CG | ||||
| · No report on AEs | ||||||
| Nielsen et al., 1996 [57], Denmark | N=38, TG: 21, CG: 17 | TG: rTMS (1 25 Hz session of 16 stimuli over the leg motor area & 1 session of 5 Hz rTMS | Spasticity: | At day 1 post-treatment: | · Significant improvement in self-score of ADLs in both groups | ⊕⊕⊝⊝ Low |
| TG: age=44 (34–67) yr, M/F=7/14, DD=12 (2–34) yr | CG: sham stimulation | AS & Achilles tendon reflex grading scores; ease of ADLs (related to spasticity) | · AS score improved significantly in TG (MD= -3.3± 4.7 AU vs. 0.7± 2.5 AU, p<0.01) | · Self-score of ease of ADLs improved significantly in both groups on day 1 post-intervention (p<0.05 for both groups), but was no difference between the two groups | ||
| CG: age=44 (26–66) yr, M/F=5/12, DD: 13 (2–30) yr | Frequency: twice daily for 7 days | Electrophysiological & biomechanical measurement: stretch reflex threshold | · Threshold of the stretch reflex significantly increased in TG (4.3±7.5 deg/s vs. -3.8±9.7 deg/s, p=0.001) | · No report on AEs | ||
| Baseline & after treatment (1 day, 8 days, & 16 days) | At day 8 post-treatment | |||||
| · Threshold of the stretch reflex remain improved significantly in TG (4.4±7.5 deg/s vs. -1.8±8.5 deg/s, p<0.05) | ||||||
| · No statistically significant difference in AS or self-score of ease of ADLs between groups | ||||||
| At day 16 post-treatment | ||||||
| · No statistically significant difference in any of the scores between two groups | ||||||
| Şan et al., 2019 [60], Turkey | N=16, TG=10, CG=6 | rTMS (5 Hz, 900 pulses over 15 minutes over vertex region | Spasticity: MAS, PSFS, Passive ROM | · Significant reductions in the MAS scores for the hip adductors bilaterally over time in TG (p<0.01), but no significant differences in CG (p>0.05) | · Significant improvements in the rTMS group patient satisfaction, amount of urine leakage, actual health status, perceived health status, energy & fatigue, role limitations due to physical problems, social function (p<0.05 for all) | ⊕⊕⊝⊝ Low |
| TG: age=48.7±14.3 yr, M/F=6/4, DD=14.7±7.7 yr | targeting lower extremity motor area) | Others: MSQOL-54, Epworth Sleepiness Scale, patient satisfaction, voiding diary | · Significant improvements in spasm (PSFS) in TG at both 1 week & 1 month (p<0.01), but no significant differences in the CG (p>0.05) | · Significant improvement in overall QOL in TG (MSQOL-54) (p<0.05) | ||
| CG: age=53.0±8.8 yr, M/F=2/4, DD=19.5±10.9 yr | CG: sham rTMS | Baseline, 1 week & 1 month after post-intervention | · No AEs or complications were observed | |||
| Frequency: 15 minutes for 10 sessions over 2 weeks | ||||||
| · | · | |||||
| Vibration therapy | ||||||
| Ayvat et al., 2021 [38], Turkey | N=33: TG I=11, TG II=11, CG=11 | TG I: local vibration 50 Hz & exercise | Spasticity: MAS | · Significant decrease in spasticity & increase in fascicle length in TG I (both p<0.05) | · Significantly improvement in ankle joint position sense, single-leg stance time, limits of stability/postural sway range in the mediolateral direction in both treatment groups (all p<0.05) | ⊕⊝⊝⊝ |
| TG I: age=37.7±9.7 yr, EDSS: 3.0±1.1, DD: 135.6±77.2 mo | TG II: local vibration 100 Hz & exercise | Ankle joint position sense: isokinetic dynamometer | · No change in TG II and CG | · Significant improvement in antero-posterior limits of stability & postural sway in all groups (all p<0.05) | Very low | |
| TG II: age=38.4±11.1 yr, EDSS: 2.75±1.0, DD= 84.0±56.9 mo | CG: exercise only | Balance: Single Leg Stance Test & post-urographic assessment | · No significant difference between groups | · TG I showed significant improvement in all walking parameters & mediolateral limits of stability | ||
| CG: age=33.9±6.7 yr, EDSS=3.0±0.8, DD=127.0±84.4 mo | Frequency: 1 hour a day for 3 sessions per week for 8 weeks | Gait: GAITRite Analysis System | · Significant improvement in velocity, step length & base of support values in the TG II (all p<0.05) | |||
| Baseline & post-treatment (8 wk) | · Between group comparisons, significant difference was found only in mediolateral limits of stability (p<0.05) | |||||
| · CG showed significant improvement only for single support & stance phase percentages of the gait cycle (both p<0.05) | ||||||
| · No report on AEs | ||||||
| Paoloni et al., 2013 [58], Italy | N=48: TG I=14, TG II=14, CG=14 | TG I: segmental muscle vibration (120 Hz) | Spasticity: MAS | · MAS score at knee & ankle significantly decreased over time (p<0.001) in all groups, but no differences between groups | · Significant reduction in fatigue (FSS scores) in vibration group & BoNT-injection only group at both 10 weeks & 22 weeks (p<0.05 for both), while no differences were detected in BoNT+vibration group | ⊕⊝⊝⊝ |
| TG I: age=54.9±8.8 yr, M/F=6/8, EDSS: 5.3 (4–5.5) | TG II: vibration & BoNT-A injection | Fatigue: FSS | · Participants not receiving BoNT-A injection only displayed a significant increase in knee & ankle spasticity at 10 weeks (p<0.05) | · Significant improvement in spasticity in all participants | ||
| TG II: age=47.4±5.6 yr, M/F=5/9, EDSS: 4.8 (3.5–5.5) | CG: BoNT-A injection | ADLs: BI | · No differences in disability (BI) over time | Very low | ||
| CG: age=50.6±8.9 yr, M/F=7/7, EDSS: 5.5 (4–6) | Frequency: 30 min 3 times per week, over 4 weeks | Baseline, 10 weeks & 22 weeks | · No adverse events | |||
| Schyns et al., 2009 [24], UK | N=16, TG I=8, TG II=8 | TG I: WBV 40 Hz, low amplitude (2 mm) for 30 seconds & stretching+strengthening exercise | Spasticity, MAS, MSSS-88 | · No change in MAS scores for either intervention | · Significant improvement in MSSS-88 scores pain (p=0.036) | ⊕⊝⊝⊝ |
| Cross over design | TG II: treatment in reverse order to TG I | Function: 10MWT, TUG | · Tone tended to increase more for exercise alone compared with whole body vibration & exercise | · No statistically significant changes for other MSSS-88 components (ADL, social functioning, stiffness, gait, body movement & emotional health) | Very low | |
| TG I: age=45.8±8.4 yr, M/F=3/5, DD: 6.7 yr (10 mo–23 yr) | Frequency: 3 times/week for 4 weeks, 2 weeks no intervention & then 4 weeks of exercise alone | Muscle force: dynamometer | · MSSS-88 spasm: greater reduction in score in TG compared CG (p<0.05, 95% CI=2.00, 14.50) | · No effects on sensation | ||
| TG II: age=49.5±6.14 yr, M/F=1/7, DD=11.8 (3.5–11.8) yr | Sensation & proprioception: Nottingham sensory Assessment | · Walking: both interventions increased the subjects’ walking speed, no difference between groups (10MWT, p>0.05), (TUG, p>0.05) | ||||
| HRQOL: MSIS-29 | · MSIS-29: overall well-being improved in both groups, no statistically significant difference between groups (p<0.05) | |||||
| Baseline & after treatment (4 wk) twice with 2 weeks cool off period | · No report on AEs | |||||
| Educational/self-management programs | ||||||
| Ehling et al., 2017 [43], Austria | N=94: TG=47, CG=47 | Both groups received 4 weeks of inpatient MDR | Spasticity: NRS, MAS, Spasm Frequency Score | · No change in MAS scores | · No difference in QOL, strength, pain, fatigue & cognition | ⊕⊝⊝⊝ |
| 2 phases: open label followed by RCT | TG: ‘MS-spasticity APP’-based exercises program | Muscle strength: Motricity Index | · At 12 weeks, significant reduction in TG (NRS MD=1.2, p<0.05) | |||
| TG: age=46.6 (43.2–50.1) yr, M/F=5/5, SPMS: 8, RRMS: 2, EDSS=4.2 (3.1–5.3), DD: 12.6 (8.8–16.5) yr | CG: paper-based exercise program for 3 months, after 3-months all received MS-spasticity-based program for another 3 months | Function: T25FWT, | · At 24 weeks, “MS-spasticity APP” was associated with a decrease in spasticity (NRS scores) in all participants (MD=2.5±1.7) | Very low | ||
| CG: age=50.5 (44.6–56.5) yr, M/F=6/4, SPMS: 9, RRMS: 1, EDSS: 5.4 (4.2–6.5), DD: 16.3 (10.6–22.0) yr | Others: self-rating scale for QOL, cognition, pain, fatigue | |||||
| Baseline, 12 weeks & 24 weeks | ||||||
| Ehling et al., 2022 [44], Austria | N=94: TG=47, CG=47 | MDR-inpatient (phase A) followed by TG: MS-Spasticity | Spasticity: NRS, MAS, Spasm Frequency Score | · Significant reduction in spasticity after MDR (NRS) (p<0.000), MAS (p<0.001), Spasm Frequency Score (p=0.001) | · MDR was also associated with significant improvements strength of lower extremities (p<0.001), & all mobility outcome measures (p<0.001) | ⊕⊝⊝⊝ |
| TG: age=50.8 (41.8–57.9) yr, M/F=18/29, SPMS:13, RRMS: 26, PPMS: 8, EDSS: 5.0 (4–6), DD (median, IQR): 13.3 (10.1–22.7) yr | App delivered CG: paper-based | Function: Motricity Index | · Superior effects on spasticity in the TG (median NRSs difference=-1.0, 95% CI=-1.7 to -0.3, p<0.01) | MS-Spasticity App was associated with: | ||
| CG: age=46.4 (41.7–55.5),; M/F=14/33, SPMS: 21, RRMS: 120, PPMS: 6, EDSS: 6.0 (4.5–6.5), DD: 12.5 (9.7–20.1) yr | exercise self-training program over 12 weeks (phase B) | Balance: FSST | · Some improvement in balance (FSST) & walking distance (2MWD) | Very low | ||
| Walking: T25FWT, 2MWD | · Significant reduction in cognitive fatigue (p<0.05), but no differences in levels of physical fatigue | |||||
| Fatigue: Würzburger Erschöpfungs-Inventar bei Multipler Sklerose | · Significantly higher exercise completion rate (92% vs. 72%, p<0.001) | |||||
| Cognition: HADS, SF-36 | ||||||
| Pre & post intervention | ||||||
| Hugos et al., 2017 [48], USA | N=40: TG=20, CG=20 | TG: group self-management program | Spasticity: MAS, MSSS-88 | · No significant changes in MAS between TG & CG (MD=-1.6 vs. -1.4, p=0.953) | · Significant improvement in TG in fatigue (MFIS p=0.03), depression (BDI-II, p=0.004), physical function (MSIS-29, p<0.01), & knowledge about spasticity on a written test (p<0.05) | ⊕⊝⊝⊝ |
| TG: age=52.8±12.3 yr, M/F=7/13, SPMS: 6, RRMS: 10, PPMS: 3, EDSS: 4.8±1.1, DD: 15.1±8.1 yr | CG: usual care (stretching booklet & home stretching) | Function: TUG, T25FWT, 2MWD, MSWS-12 | · Significant improvement in MSSS-88 total scores in TG compared to CG (MD=-27.8 vs. -3.7, p<0.05); on the pain & discomfort subscale (MD=-3.9 vs. +0.3, p<0.05) & muscle spasms subscale (MD=-5.0 vs. -0.5, p<0.05) | · No significant group difference (p>0.05) | ||
| CG: age=53.4±12.8 yr, M/F=4/16, SPMS: 4, RRMS: 8, PPMS: 7, EDSS: 4.9±1.5, DD: 15.7±10.5 yr | Frequency: 2 hourly session & daily stretch for 4 weeks | Cognition: MSIS-29, BDI-II | · No significant changes in physical tests | Very low | ||
| Pre & post intervention | ||||||
| tDCS | ||||||
| El Habashy et al., 2022 [45], Egypt | N=20: TG=10, CG=10 | TG: anodal tDCS | Spasticity: MAS, H latency, H/M amplitude ratio | · No significant differences in the MAS scores in both groups (p=0.22) | · No report on AEs | ⊕⊝⊝⊝ Very low |
| TG: age=30±6.53 yr, EDSS=4.15±1.31, DD=3.70±3.09 yr | CG: sham tDCS | Before & after treatment (5 days) | · H/M amplitude ratio: TG showed significant improvement (0.6±0.2 vs. 0.5±0.1), no change in CG (0.6±0.2 vs. 0.6±0.2) | |||
| CG: age=3.0±9.8 yr, EDSS=4.15±0.91, DD=4.30±3.47 yr | Frequency: 1/daily session of 20-minute stimulation, 5 consecutive days | · H latency: no difference in TG (30.8±2.8 vs. 30.3±2.6), but significant decreased in CG (32.9±2.6 vs. 31.9±2.6) | ||||
| Iodice et al., 2015 [50], Italy | N=20: TG=10, CG=10 | TG: anodal tDCS (2 mA to primary motor cortex of the more affected side) | Spasticity: MAS, MSSS-88 | · No significant interaction for spasticity scales: MAS (p>0.05); MSSS-88 (p>0.05) | · No significant changes in walking in both groups (MSWS-12, p>0.05) | ⊕⊝⊝⊝ Very low |
| TG: age=43.3±7.5 yr, M/F=2/8, EDSS: 3.6±0.9, DD=7.0±3.1 yr | CG: sham tDCS | Function: MSWS-12 | · No significant differences between the two groups post-intervention: MAS, MSSS-88 (p>0.05 for both ) | · No group difference (MSWS-12, p>0.05) | ||
| CG: age=40.3±4.5 yrs, M/F=3/7, EDSS: 3.8±0.9, DD=7.8±1.9 yr | Frequency: 20 min/day for 5 days | Baseline & post-intervention (5 days) | · No AEs | |||
| Pulsed electromagnetic device | ||||||
| Lappin et al., 2003 [53], USA | N=117 multisite cross-over design, all underwent treatment device alternating with control device and vice versa | TG: PEM device | MSQLI: fatigue, pain, spasm/spasticity, bladder control, QOL | · No significant reduction in spasticity | · Significantly greater Improvement in fatigue & overall QOL | ⊕⊝⊝⊝ |
| Age (range)=21–64, M/F=28/89, SPMS: 27, RRMS: 52, PPMS: 12, DD (range)=1 to >13 yr | CG: placebo | Function: MS Performance scales; MS Rating Form and Mobility Index | · Significant improvement in spasms (MD=-0.13; p<0.05) | · No significant differences in the treatment effects for bladder control (p>0.05) (MSQLI) & disability composite (p>0.05) (MSPS) | Very low | |
| Frequency: 4 weeks for up to 24 h/day), separated by a 2-week washout period | · No further data or analysis reported for spasms/spasticity | · 3 scales (fatigue, pain & spasticity) used to create the QOL index (QLI) showed moderate inter-correlations (r=0.32 to 0.60), however, bladder control scale showed poor correlation with other MSQLI scales (r=0.00 to 0.26) | ||||
| Baseline, after 4-week treatment & 10 weeks | · No report on AEs | |||||
| Richards et al., 1997 [59], USA | N=30: TG=15, CG=15 | TG: magnetic pulsing device (Enermed), range: 4–13 Hz (50–100 milliCauss) | Patient-reported performance scale-symptoms: spasticity, bladder control, cognitive level, fatigue level, hand function, mobility, sensation, vision | · Significant difference between pre-treatment & post treatment within the treatment group in spasticity: MD=-0.80±0.23; p<0.01 | · No significant change in EDSS scale | ⊕⊝⊝⊝ |
| TG: M/F=8/11, SPMS or PPMS: 8, RRMS: 7, EDSS: 5.1 (0–9) | CG: sham device | Function: EDSS brain electric activity: quantitative EEG during a language task) | · No difference between group | · Significant improvement in the performance scale combined rating for: bladder control, cognition, fatigue, mobility, vision (p<0.05 for all) | Very low | |
| CG: M/F=5/10, SPMS or PPMS: 10, RRMS: 5, EDSS: 4.98 (0–8.5) | Frequency: 10–24 hours a day for 2 months | Baseline & after treatment (2 mo) | · Significant change between pre-treatment & post-treatment in alpha EEG magnitude during the language task | |||
| · 19 AEs reported (11 in intervention group & 8 in control group) | ||||||
| TENS | ||||||
| Miller et al., 2007 [22], UK | N=32: TG=16, CG=16 | TENS (100 Hz, 0.125 ms pulse width)-60 minutes vs. 8 hours | Baseline & after (2 wk) treatments; & follow-up (8–20 mo) for the questionnaires for patient report for symptoms | · No statistically significant differences in the Global Spasticity Score following either 60 minutes or 8 hours daily of TENS (p>0.05 for both) | · At the end of the study (8–20 mo) patients reported reduction in symptoms: 87.5% for spasm, 73.3% for pain & 73.3% for stiffness | ⊕⊝⊝⊝ |
| Cross-over design | Frequency: 2 weeks of 60 minutes (period A) & 8 hours daily (period B) followed by 2 weeks washout period | · 8-hour TENS compared with 60 minutes led to significant reduction in muscle spasm (p<0.05) & pain (p<0.01) | · No report on AEs | Very low | ||
| Demographic not reported | ||||||
| Shaygannejad et al., 2013 [61], Iran | N=58: TG=28, CG=30 | TG: TENS (100 Hz, with pulse width set at 250 ps) 20–30 minutes | Spasticity: MAS | · Significant reduction in MAS score at 4 weeks in both groups (TG MD=-1.04, CG: MD=-0.58, p<0.001 for both) | · Four participants from the baclofen group dropped out due to AEs | ⊕⊝⊝⊝ |
| TG: age=39.5±9.3 yr, M/F=9/17, SPMS: 5, RRMS: 20, PPMS: 1, EDSS: 2.1±1.4, DD: 7.2±5.0 yr | CG: baclofen (10 mg twice daily, increasing over 3 weeks to 25 mg) | Baseline & 4 weeks | · MAS scores significantly lower in TG at 4 weeks than CG (MD=-0.42, p<0.05) | Very low | ||
| CG: age=38.9±7.8 yr, M/F=6/12, SPMS: 8, RRMS: 18, PPMS: 0, EDSS: 2.6±1.3, DD: 5.3±2.8 yr | Frequency: 4-week | |||||
| Shock wave therapy | ||||||
| Marinelli et al., 2015 [54], Italy | N=68: TG=34, CG=34 | TG: RSWT over ankle extensor muscles (4 Hz frequency, with a pressure of 1.5 Bars, 2,000 shots) | Spasticity: MAS | · MAS scores significantly decreased only at 1 week (MD=-0.78, p<0.0001) | · Significant reduction in pain VAS scores at all follow-up assessments, with the maximal effect at 1 week (MD= 3.05, p<0.0001) | ⊕⊝⊝⊝ |
| TG: age=51.7± 11.3 yr, M/F=14/20, EDSS: 6.6± 0.8 | CG: placebo RSWT | Spinal excitability: H-reflex | · No changes at 4 weeks | · No significant changes in ankle strength & walking | ||
| CG: age=51.00±13.2 yr, M/F=16/18, EDSS: 6.2±1.2 | Frequency: 4-session course, with a 1-week interval between sessions | Pain: VAS | · No significant changes in H-reflex compared to healthy controls | · Spinal excitability was unaffected | Very low | |
| Ankle strength: Medical Research Council rating | · No significant changes in any of the parameters in CG | |||||
| Walking: 10MWT | · No AEs | |||||
| Baseline; 1 week after the first session; & 4 weeks after last session | ||||||
| Complementary and alternative medicine | ||||||
| Karpatkin et al., 2023 [51], USA | N=12: TG=6, CG=6 | TG: acupuncture CG: no treatment | Spasticity: MAS | · Significant improvement in spasticity (MAS) score in right hip flexors (p<0.05) | · No statistically significant changes were observed in the gait or balance measures | ⊕⊝⊝⊝ |
| Crossover design | Frequency: twice weekly for 4 weeks, followed by a 1-week washout period, & then crossed over to the other condition for 4 weeks | Gait & balance: 6MWT, T25FWT, Mini-Balance Evaluation System Test | · Other lower limb muscles were unaffected (p>0.05 for all) | · Small statistically significant changes were observed in upper extremity strength | Very low | |
| Age=52.7±16.3 yr, M/F=6/6, SPMS: 2, RRMS: 9, PPMS: 1, EDSS: 3.4±0.76 | Strength: handheld dynamometer | · No changes in sensation | ||||
| Sensory testing: Biothesio meter | · No AEs | |||||
| Baseline & post intervention | ||||||
GRADE, Grading of Recommendations Assessment, Development and Evaluation; TG, treatment group; CG, control group; M/F, male/female; EDSS, Expanded Disability Status Scale; DD, disease duration; MS, multiple sclerosis; RRMS, relapsing-remitting MS; DNS, dynamic neuromuscular stabilization; CS, core stabilization exercises; MSSS-88, MS Spasticity Scale-88; MAS, Modified Ashworth Scale; BBS, Berg Balance Scale; MSWS, MS Walking Scale; TUG, Timed Up and Go; AE, adverse event; sEMG, surface electromyography; ES, effect size; IG, intervention group; RAGT, robot-assisted gait training; VR, virtual reality; FIM, Functional Independence Measure; COPE, Coping Orientation to Problem Experienced; HRSD, Hamilton Rating Scale for Depression; CI, confidence interval; MMT, Manual Muscle Test; FRI, Fall Risk Index; OSI, Overall Stability Index; SSE, static stretching exercise; FSE, functional stretching exercise; T25FWT, Timed 25-Foot Walk Test; ROM, range of motion; VAS, visual analogue scale; QOL, quality of life; HRQOL, health-related QOL; BoNT, botulinum toxin; PT, physiotherapy; MD, mean difference; SD, standard difference; PPMS, primary progressive MS; STC, Spasticity: Take Control; NRS, Numeric Rating Scale; MFIS, Modified Fatigue Impact Scale; PSQI, Pittsburgh Sleep Quality Index; PROMIS, Patient-Reported Outcomes Measurement Information System; MSIS, MS Impact Scale; 10MWT, 10-Meter Walk Test; MSQLI, MS QOL Inventory; FSS, Fatigue Severity Scale; MSQOL-54, Multiple Sclerosis QOL-54 Scale; MSIQOL, MS International QOL; NAB, neuropsychological assessment battery; TOL, Tower of London Test; CES-D, Center for Epidemiologic Studies Depression Scale; MSQOL, MS QOL Scale; 6MWT, 6-Meter Walking Test; ADS, Allgemeine Depressionsskala; SPMS, secondary progressive MS; iTBS, intermittent theta burst stimulation; fMRI, functional magnetic resonance imaging; PCS, cortical silent period; rTMS, repetitive transcranial magnetic stimulation; SESS, Subjective Evaluating Spasticity Scale; NAS, Numerical Analog Scale; AS, Ashworth Scale; ADLs, activities of daily living; BI, Barthel Index; PSFS, Penn Spasm Frequency Scale; WBV, whole body vibration; RCT, randomized controlled trial; MDR, multi-disciplinary rehabilitation; FSST, Four Square Step Test; 2MWD, 2 meter walking distance; HADS, Hospital Anxiety and Depression Scale; SF-36, Short Form-36; BDI-II, Beck Depression Inventory; tDCS, transcranial direct current stimulation; MSPS, MS Performance Scale; EEG, electroencephalogram; TENS, transcutaneous electric nerve stimulation; RSWT, radial shock wave therapy.
Table 2.
| Reference | Bias risk | Inconsistency | Indirectness | Imprecision | Publication bias | GRADE |
|---|---|---|---|---|---|---|
| Abadi Marand et al., 2023 [36] | + (-1) | NS | NS | + (-1) | U | Low (+2) |
| Andreu-Caravaca et al., 2022 [37] | + (-1) | NS | + (-1) | NS | U | Low (+2) |
| Ayvat et al., 2021 [38] | ++ (-2) | NS | NS | + (-1) | U | Very low (+1) |
| Boutière et al., 2017 [39] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Calabrò et al., 2017 [40] | + (-1) | NS | NS | + (-1) | U | Low (+2) |
| Dieguez-Varela et al., 2019 [41] | + (-1) | NS | + (-1) | NS | U | Low (+2) |
| Eftekharsadat et al., 2015 [42] | ++ (-2) | NS | NS | NS | U | Low (+2) |
| Ehling et al., 2017 [43] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Ehling et al., 2022 [44] | ++ (-2) | NS | NS | + (-1) | U | Very low (+1) |
| El Habashy et al., 2022 [45] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Ergül et al., 2021 [46] | + (-1) | NS | NS | + (-1) | U | Low (+2) |
| Giovannelli et al., 2007 [47] | ++ (-2) | NS | NS | NS | U | Low (+2) |
| Hugos et al., 2017 [48] | ++ (-2) | NS | NS | + (-1) | U | Very low (+1) |
| Hugos et al., 2024 [49] | ++ (-2) | NS | NS | NS | U | Low (+2) |
| Iodice et al., 2015 [50] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Karpatkin et al., 2023 [51] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Korzhova et al., 2019 [52] | +1 (-1) | NS | NS | NS | U | Moderate (+3) |
| Lappin et al., 2003 [53] | + (-1) | + (-1) | NS | + (-1) | U | Very low (+1) |
| Marinelli et al., 2015 [54] | + (-1) | + (-1) | NS | + (-1) | U | Very low (+1) |
| Miller et al., 2007 [22] | ++ (-2) | + (-1) | NS | NS | U | Very low (+1) |
| Mori et al., 2011 [55] | + (-1) | NS | + (-1) | NS | U | Low (+2) |
| Negahban et al., 2013 [56] | + (-1) | NS | NS | NS | U | Moderate (+3) |
| Nielsen et al., 1996 [57] | + (-1) | + (-1) | NS | NS | U | Low (+2) |
| Paoloni et al., 2013 [58] | + (-1) | + (-1) | NS | + (-1) | U | Very low (+1) |
| Richards et al., 1997 [59] | + (-1) | + (-1) | + (-1) | + (-1) | U | Very low (0) |
| Şan et al., 2019 [60] | ++ (-2) | NS | NS | NS | U | Low (+2) |
| Schyns et al., 2009 [24] | ++ (-2) | NS | + (-1) | NS | U | Very low (+1) |
| Shaygannejad et al., 2013 [61] | ++ (-2) | + (-1) | NS | + (-1) | U | Very low (0) |
| Tarakci et al., 2013 [62] | + (-1) | NS | NS | NS | U | Moderate (+3) |
| Velikonja et al., 2010 [63] | ++ (-2) | + (-1) | + (-1) | NS | U | Very low (0) |
| Vermöhlen et al., 2018 [64] | ++ (-2) | + (-1) | NS | NS | U | Very low (+1) |
| Zrzavy et al., 2021 [65] | ++ (-2) | NS | NS | NS | U | Low (+2) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download