Abstract
Background
Thyroid-associated ophthalmopathy (TAO) involves tissue expansion and inflammation, potentially causing a hypoxic microenvironment. Hypoxia-inducible factor (HIF)-1α is crucial in fibrosis and adipogenesis, which are observed in TAO progression. We investigated the effects of hypoxia on orbital fibroblasts (OFs) in TAO, focusing on the role of HIF-1α in TAO progression.
Methods
OFs were isolated from TAO and non-TAO patients (as controls). In addition to HIF-1α, adipogenic differentiation markers including peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein (CEBP) were measured by Western blot, and phenotype changes were evaluated by Oil Red O staining under both normoxia and hypoxia. To elucidate the effect of HIF-1α inhibition, protein expression changes after HIF-1α inhibitor treatment were evaluated under normoxia and hypoxia.
Results
TAO OFs exhibited significantly higher HIF-1α expression than non-TAO OFs, and the difference was more distinct under hypoxia than under normoxia. Oil Red O staining showed that adipogenic differentiation of TAO OFs was prominent under hypoxia. Hypoxic conditions increased the expression of adipogenic markers, namely PPARγ and CEBP, as well as HIF-1α in TAO OFs. Interleukin 6 levels also increased in response to hypoxia. The effect of hypoxia on adipogenesis was reduced at the protein level after HIF-1α inhibitor treatment, and this inhibitory effect was sustained even with IGF-1 stimulation in addition to hypoxia.
Thyroid-associated ophthalmopathy (TAO) is a significant extrathyroidal manifestation and comorbidity, predominantly associated with Graves’ disease (GD) and, to a lesser extent, found in euthyroid or hypothyroid patients who have thyroid autoantibodies. Approximately half of the patients with GD exhibit symptoms and signs indicative of TAO, including abnormal ocular sensations, increased lacrimation, eyelid edema or retraction, and conjunctival injection [1]. Imaging techniques such as magnetic resonance imaging or computed tomography scans detect TAO in about 70% of GD patients, encompassing both subclinical and clinical cases [1]. TAO is more prevalent among women than men and is more common in the White population than in the Asian population [2]. While mild cases of TAO typically do not require treatment beyond that for GD, moderate to severe cases, which represent 3% to 5% of TAO instances, necessitate specific targeted therapies [1]. In the most severe cases of TAO, complications such as optic neuropathy or corneal injury may occur, potentially leading to vision loss.
The underlying mechanism for TAO primarily involves autoimmune processes [1,3]. The presence of thyrotropin receptor (TSH-R) in orbital fibroblasts (OFs) and their interactions with infiltrating immune cells, including T cells, B cells, and macrophages, play a crucial role in the development and progression of TAO. In the early phase of the disease, the main pathological features include inflammation with adipose tissue expansion due to adipogenic differentiation of OFs and extraocular muscle expansion through the accumulation of glycosaminoglycans. Tissue fibrosis may occur in the later stages of TAO. Currently, glucocorticoid treatment, which modulates inflammation and immune responses, is the primary therapy for TAO. Recent studies have implicated the insulin-like growth factor (IGF)-1 receptor, in conjunction with TSH-R, in the pathogenesis of TAO, and a monoclonal antibody targeting the IGF-1 receptor has demonstrated clinical benefits [4]. However, the molecular and cellular mechanisms of TAO remain incompletely understood, and existing treatment strategies are inadequate to halt the progression of the disease, despite the significant systemic adverse effects associated with these medications.
In addition to autoimmune processes that initiate and exacerbate TAO, tissue hypoxia has also been suggested as a potential link between smoking and TAO, according to some studies [5-7]. The development of a hypoxic microenvironment in TAO can be explained by factors such as inflammation [8] and the expansion of adipose tissue, which may induce hypoxia [9]. Given the anatomical constraints of the orbit’s bony structure, any expansion of tissue within this limited space can lead to vascular compression and reduced blood flow, as observed in TAO [10,11]. If the specific roles of the hypoxic microenvironment in the progression of TAO are identified, it could lead to the development of new management strategies that complement existing treatments, which primarily focus on immune suppression.
Hypoxia-inducible factor (HIF)-1α, a crucial molecule in hypoxic conditions, significantly influences adipogenesis and fibrosis by regulating cellular metabolism and the extracellular matrix, both of which are involved in the progression of TAO [1,2]. Therefore, we explore the impact of hypoxia on OFs from TAO patients, specifically examining the role of HIF-1α in adipogenesis during the progression of TAO.
OFs were obtained from the Department of Ophthalmology at Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea. TAO OFs were collected during surgeries on patients, while non-TAO OFs were derived from upper lid blepharoplasties in individuals without any history of immune, inflammatory, or thyroid diseases. The study’s protocol, including the acquisition of specimens, received approval from the Institutional Review Board of College of Medicine, The Catholic University of Korea (KC17SESI0504) and written informed consent was obtained from all patients. This research was conducted in accordance with the ethical standards of the Declaration of Helsinki for medical research involving human participants. The clinical characteristics of the primary cells are presented in Supplemental Table S1.
Primary cultures of OFs were grown in Dulbecco’s Modified Eagle’s Medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA).
OFs were seeded at a density of 5×103 cells per well in 6-well plates. They were cultured in adipogenesis medium, specifically Human Adipose-derived Mesenchymal Stem Cells Complete Medium (Cyagen, Santa Clara, CA, USA). Following a 14-day incubation, the media were discarded, and the wells were washed three times with phosphate-buffered saline (PBS). Each well was then fixed with 10% formaldehyde for 10 minutes, followed by a rinse with distilled water. Subsequently, each well was stained with a 0.3% Oil Red O solution in distilled water and isopropanol for 20 minutes. After staining, the wells were washed with a 60% isopropanol solution. Finally, the wells were left to dry overnight on a clean bench and were examined under a microscope (Nikon TS-100F) at 400× magnification.
OFs were washed three times with PBS and lysed using radioimmunoprecipitation assay (RIPA) lysis buffer (Bio-Rad, Hercules, CA, USA) supplemented with a protease inhibitor cocktail (Bio-Rad). Protein samples, each containing 50 μg, were prepared using the bicinchoninic acid method with a bovine serum albumin standard and subsequently loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. After electrophoresis, the proteins were transferred to membranes (Millipore, Bedford, MA, USA), and Ponceau S staining (Abcam, Cambridge, UK) was employed to assess the efficiency of the transfer. The membranes were then blocked overnight in a solution of 5% skim milk and 5% bovine serum albumin in PBS containing 0.2% Tween 20 (Bio-Rad). This was followed by incubation with the primary antibody. After the primary antibody incubation, the membranes were probed with a horseradish peroxidase-conjugated secondary antibody and developed using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher). Protein detection was performed with the ChemiDoc MP system (Bio-Rad).
IDF-11774 (provided by Ildong Pharmaceutical Co. Ltd., Seoul, Korea), previously known as Lw6, is an HIF-1α inhibitor that has demonstrated effectiveness in treating colorectal, lung, and thyroid cancers [12-14]. Cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) assay. Cells were treated with varying concentrations of IDF-11774 (0, 0.5, 1.0, 1.5, 2.0, and 2.5 μM) and incubated at 37°C in a 5% CO2 atmosphere for 48 hours. Following this, 10 μL of MTT solution was added to each well. After incubation for 4 hours in a 5% CO2 environment at 37˚C, the medium was removed from all wells. Isopropyl alcohol was added to each well, followed by a 20-minute incubation period, after which distilled water was added. The absorbance at 570 nm was measured using a Tecan Infinite M200 Pro microplate reader (Tecan Life Sciences, Männedorf, Switzerland). The half-maximal inhibitory concentration (IC50) was determined as the concentration that reduced the absorbance in normal cells by 50% in the MTT assay, indicating a 50% reduction in cell viability.
A hypoxia chamber was utilized to create hypoxic conditions, under which the cultured dishes were handled and treated. The cells were incubated at 37°C in an atmosphere containing 5% CO2. Hypoxic conditions were established using a gas mixture of 1% O2, 5% CO2, and 94% N2 at a constant temperature of 37°C over a 48-hour period in an incubator chamber (Baker Ruskinn, Bridgend, UK). OFs were not used beyond passage number 8 in the initial culture.
We categorized experimental conditions into six groups to distinguish between normoxic and hypoxic environments. Further subdivisions were based on IDF-11774 treatment, allowing for a detailed analysis of the effects on adipogenesis. To enable focused analyses, we created data subsets based on specific samples aligned with each sample group and condition. To visually depict the distribution of protein expression values across various conditions, we employed boxplots demonstrating the median, quartiles, and outliers. For comparing protein expression levels in normoxic versus hypoxic conditions across each sample group, we utilized the Wilcoxon signed-rank test. A nonparametric test for paired data was applied to assess proteins like HIF, CCAAT/enhancer binding protein (CEBP), and peroxisome proliferator-activated receptor γ (PPARγ) in each sample. Protein expression values were represented using the median and interquartile range. We performed two-way analysis of variance (ANOVA) to evaluate the effects of group (TAO vs. non-TAO), condition (normoxic vs. hypoxic), and their interaction on protein expression values. To strengthen the robustness of our findings, we used bootstrapping analysis to validate the F values and P values obtained from two-way ANOVA. Additionally, we conducted one-way ANOVA to evaluate the variation in protein expression and cytokines across different treatment conditions. After ANOVA, the Tukey honest significant difference post hoc test was conducted to evaluate the significance of differences between groups. Statistical analyses were performed using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was defined as a P value less than 0.05.
To investigate HIF-1α expression in OFs, primary cells from TAO patients and non-TAO controls were cultured under normoxic (5% CO2 in air) and hypoxic conditions. Fig. 1 illustrates the HIF-1α expression levels in each sample under both normoxia and hypoxia. Two-way ANOVA revealed significant main effects for both group and condition, as well as a significant interaction effect. TAO OFs exhibited significantly higher HIF-1α expression than non-TAO OFs (F(1, 32)=332.012, P<0.001, η2=0.735). Additionally, hypoxia led to a significant increase in HIF-1α expression compared to normoxia (F(1, 32)=93.189, P<0.001, η2=0.206). The interaction effect indicated that the increase in HIF-1α expression under hypoxia was more pronounced in TAO OFs than in non-TAO OFs (F(1, 32)=14.779, P<0.001, η2=0.033). Bootstrapped F values confirmed the robustness of these findings, with mean values of 403.0745 for the group effect, 128.1350 for the condition effect, and 16.92281 for the interaction effect, further supporting the observation of significant differences. These results suggest that HIF-1α expression is significantly higher in TAO OFs, especially under hypoxic conditions.
Phenotypically, TAO OFs cultured in adipogenic medium for 14 days exhibited adipogenic differentiation (Fig. 2). Under hypoxic conditions, more prominent lipid droplets were observed compared to those under normoxic conditions.
As illustrated in Fig. 3, all TAO samples exhibited higher HIF1α expression under hypoxic conditions. Additionally, CEBP and PPARγ expression levels were elevated in hypoxic conditions compared to normoxic conditions. Notably, TAO 144 was the sample that demonstrated the most significant increase in protein expression in response to hypoxic stimuli, with statistical significance.
Next, we investigated whether the expression of HIF-1α, CEBP, and PPARγ could be affected by inhibiting HIF-1α using IDF-11774 (IC50=58.6 and 51.5 μM in normoxia and hypoxia, respectively) (Supplemental Fig. S1). We observed a decrease in the expression of HIF-1α, CEBP, and PPARγ with increasing concentrations of IDF-11774 (15 and 30 μM) as shown in Fig. 4. There was a statistically significant difference in the expression levels of all proteins among the no-treatment group, the IDF-11774 15 μM group, and the IDF-11774 30 μM group. In post hoc analysis, a significant decrease in the expression of HIF-1α, CEBP, and PPARγ was noted between the no-treatment group and the IDF-11774 30 μM group (P=0.011, P=0.002, and P=0.004, respectively).
To further investigate whether the HIF-1α inhibitor elicited a similar response under IGF-1 stimulation as it did under hypoxia alone, TAO OFs were exposed to IGF-1 (20 mM) under hypoxic conditions, followed by treatment with the HIF-1α inhibitor. Stimulation with IGF-1 resulted in increased expression of HIF-1α, CEBP, and PPARγ. However, the presence of the HIF1α inhibitor significantly reduced the expression of these markers, even in the presence of IGF-1 (Fig. 5). Specifically, the expression of HIF-1α showed a borderline significant decrease (P=0.06), CEBP expression demonstrated a significant reduction (P=0.007), and PPARγ expression did not exhibit a significant change (P=0.5530).
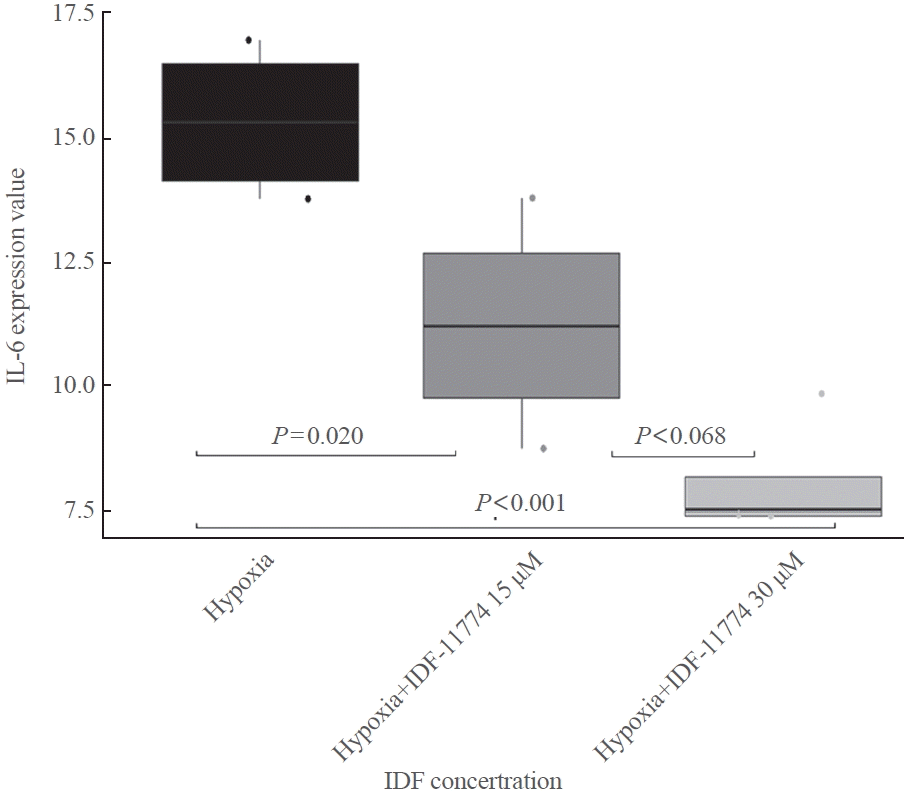
We analyzed the effects of hypoxia and HIF-1α inhibition on inflammatory cytokines, including tumor necrosis factor α, interleukin 1β (IL-1β), IL-2, and IL-6. Hypoxia significantly increased IL-6 levels (Supplemental Fig. S2), and this elevation was substantially reduced by the HIF-1α inhibitor (Supplemental Fig. S3). Additionally, the IL-6 response to the HIF-1α inhibitor was evident when IGF-1 stimulation was introduced under hypoxic conditions (Fig. 6). ANOVA revealed a significant effect of IDF-11774 concentration on IL-6 expression levels (P<0.001). In the post hoc analysis, IL-6 expression at an IDF-11774 concentration of 15 μM was significantly lower than that of the control (P=0.020). Similarly, IL-6 expression at an IDF-11774 concentration of 30 μM was significantly lower than that of the control (P<0.001). However, there was no significant difference in IL-6 expression between IDF-11774 concentrations of 30 μM and 15 μM (P=0.068).
In this study, we observed prominent HIF-1α expression and a robust increase in response to hypoxic stimuli in TAO OFs. Under hypoxic conditions, TAO OFs exhibited changes to an adipogenic phenotype. Additionally, the expressions of adipogenic differentiation markers in TAO OFs, specifically CEBP and PPARγ, were more pronounced under hypoxia than under normoxic conditions. Most importantly, inhibition of HIF-1α significantly downregulated adipogenic markers (CEBP and PPARγ) and the inflammatory cytokine IL-6, highlighting its potential role in modulating adipogenesis and inflammation in TAO OFs.
HIF-1α is a main regulator in response to hypoxic stimuli under both physiological and pathological conditions. In normoxia, HIF-1α is located in the cytoplasm and is rapidly degraded by the proteasome, resulting in undetectable levels of HIF-1α [15]. In contrast, under hypoxic conditions, HIF-1α stabilizes and translocates from the cytoplasm to the nucleus. It then dimerizes with HIF-1β, initiating gene transcription that enables cells to adapt and survive under hypoxic conditions [16]. HIF1α activates the transcription of genes involved in various cellular processes, including angiogenesis (vascular endothelial growth factor [VEGF]) [17,18], epithelial fibrosis (plasminogen activator inhibitor-1) [19], glucose metabolism (oxygen-dependent tricarboxylic acid cycle and glucose transporters) [20], iron metabolism and erythropoiesis (transferrin and transferrin receptor) [21], cell proliferation (IGF and transforming growth factor-α) [22], and apoptosis (p53 and p21) [23]. Consequently, tissues must adapt to changes in oxygen concentration, and various targets of HIF-1α are regulated in a tissue-specific manner [16]. Although HIF-1α is typically rapidly degraded under normoxic conditions, it can still be detected in certain situations. Basal cellular activities may result in low levels of HIF-1α even in the presence of normal oxygen levels. Moreover, specific cell types, such as cancer cells [24,25], or external conditions, such as inflammation [26,27], can stabilize or accumulate HIF-1α despite adequate oxygen availability.
The complex interplay between the immune system and OFs is known to play a crucial role in the initiation and progression of TAO [1]. Recent studies have also implicated the IGF-1 pathway in the pathogenesis of TAO [28]. Moreover, based on clinical and laboratory evidence, hypoxia is likely to contribute to the progression of TAO. Firstly, smoking, which can induce tissue hypoxia or create a hypoxic microenvironment [6], is a well-known risk factor for both the development and exacerbation of TAO. Secondly, the expansion of tissue within the confined bony orbit in TAO could lead to a hypoxic microenvironment. Lastly, hypoxia has been shown to play a significant role in the adipogenic differentiation of fibroblasts observed in OFs of TAO patients [6,7,29]. In light of these findings, we hypothesize that HIF-1α, a primary regulator in response to hypoxic conditions, plays a significant role and could be an effective target for treatment.
To date, limited data demonstrate the role of hypoxia in the progression of TAO. Metcalf and Weetman [5] showed that OF responded to cytokine (IL-1 and interferon gamma) stimulation under hypoxic conditions. However, their experiments were conducted only on OFs from healthy subjects. Another study identified differences in adipogenesis under hypoxic conditions between TAO OFs and controls [6]. Gortz et al. [7] investigated the HIF-1-dependent VEGF and adipogenic differentiation in TAO OFs, noting increases associated with smoking. Recent research has furthered this understanding by demonstrating that the interaction between macrophages and OFs under hypoxic conditions not only perpetuates inflammation but also significantly enhances adipogenic differentiation, suggesting a compounding effect on the pathogenesis of TAO that could inform new therapeutic strategies [29]. Van Regemorter et al. [30] found that the expression of HIF-1α protein was increased in TAO adipose tissues but not in TAO OFs. We also observed that HIF-1α expression levels in TAO OFs were higher under normoxia than those in non-TAO OFs and significantly increased under hypoxia only in TAO OFs. In our study, adipogenesis, measured by Oil Red O staining, was increased in TAO OFs under hypoxia. Adipogenesis is associated with the activation of several adipocyte-specific genes such as adiponectin and leptin [31]. PPARγ and CEBPβ stimulate adiponectin and act as factors for terminating the differentiation of fibroblasts into adipocytes [32]. A previous study reported that PPARγ is expressed in the orbital adipose tissue of five TAO patients and is stimulated in cultures of TAO with treatment of a PPARγ agonist (thiazolidinediones) [33]. Our study also confirmed a consistent result of increased PPARγ and CEBP levels under hypoxia in TAO OFs, while those adipogenic marker expressions decreased after a dose-dependent IDF-11774 treatment. Additionally, among inflammatory cytokines, IL-6 responded to hypoxic stimuli and HIF-1α inhibition. However, while the effect of HIF-1α inhibition on IL-6 was maintained with IGF-1 stimulation under hypoxia, the effect on HIF-1α expression was attenuated and that on PPARγ expression was not significant. Therefore, HIF-1α inhibition might not be a good candidate for single therapy of TAO.
Our findings have several clinical implications. First, in conjunction with prior studies [5-7,29], HIF-1α, which is induced by microenvironmental changes such as hypoxia, could be a new target for preventing the progression of TAO. Second, the microenvironment, which is known to play a significant role in cancer progression, could also be crucial in benign diseases such as TAO [20]. Therefore, manipulating aspects of the microenvironment beyond hypoxia might represent another strategic approach for managing benign diseases. Lastly, since the HIF-1α-related mechanism in TAO progression operates independently from immune processes, combining these treatments could be a viable option. This approach could enhance treatment effectiveness, as demonstrated in rheumatoid arthritis [34]. By introducing a new treatment target apart from the immune system, it may be possible to reduce steroid doses and thereby decrease the risk of complications. Furthermore, a strength of this study lies in the fact that all laboratory procedures under hypoxic conditions were conducted within a hypoxic chamber. In contrast, previous studies did not consistently perform all procedures within such a chamber, potentially exposing samples to oxygen.
However, there are several limitations of our study. As this study utilized primary cells from several TAO patients, further studies using various types of TAO OFs are required to generalize the findings. Additionally, it is important to note that primary cells can undergo phenotypic changes at higher passage numbers. Therefore, in our experiments, OFs were not used beyond passage number 8 in the initial culture. This precaution should be considered in future TAO studies to maintain consistent cellular characteristics. The proposed mechanism suggests that HIF-1α appears to be involved in the progression of TAO, but not in its initial development.
In conclusion, our study has elucidated the pivotal role of HIF-1α in the progression of TAO, especially under hypoxic conditions. Importantly, the application of a HIF-1α inhibitor effectively decreased the expression of adipogenic markers, underscoring the potential of targeting HIF-1α as a therapeutic strategy in TAO management.
Supplementary Material
Supplemental Table S1.
Clinical Characteristics of TAO Samples
Supplemental Fig. S1.
Determination of the half-maximal inhibitory concentration (IC50) of IDF-11774, a hypoxia-inducible factor (HIF)- 1α inhibitor, under normoxic and hypoxic conditions. (A) The IC50 of IDF-11774 in orbital fibroblasts under normoxic conditions was determined to be 58.62 μM. Cell viability was assessed after treatment with increasing concentrations of IDF-11774. (B) The IC50 of IDF-11774 in orbital fibroblasts under hypoxic conditions was determined to be 51.51 μM. Cell viability was similarly assessed after treatment with increasing concentrations of IDF-11774. The curves indicate a dose-dependent decrease in cell viability with increasing concentrations of IDF-11774 under both conditions, with slightly lower IC50 under hypoxia, suggesting increased sensitivity of cells to HIF-1α inhibition in hypoxic conditions.
Supplemental Fig. S2.
Cytokine expression under normoxic and hypoxic conditions. The figure shows box plots of cytokine expression values for thyroid-associated ophthalmopathy (TAO) samples (TAO 144 and TAO 172) under normoxic and hypoxic conditions. Cytokine expression levels are depicted for interleukin 1β (IL-1β), IL-2, IL-6, and tumor necrosis factor (TNF)-α. In non-parametric tests, only IL-6 levels exhibited significant differences between normoxia and hypoxia in both samples (P=0.005 for both).
Supplemental Fig. S3.
Interleukin 6 (IL-6) response to hypoxia-inducible factor (HIF)-1α inhibition under hypoxia. (A) The figure displays box plots of IL-6 expression levels under hypoxic conditions for thyroid-associated ophthalmopathy (TAO) samples at different IDF-11774 concentrations (0, 15, and 30 μM). The observations indicate a decrease in IL-6 expression with increasing IDF-11774 concentration for both cell lines, suggesting a dose-dependent response under hypoxic conditions. (B) Post hoc analysis reveals significant differences in IL-6 expression between certain IDF-11774 concentrations, with adjusted P values indicating statistical significance. CI, confidence interval.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: J.L., J.L., D.J.L., S.B.L., S.W.Y., M.H.K. Acquisition, analysis, or interpretation of data: J.L., J.L., D.J.L., S.B.L., S.W.Y., M.H.K. Drafting the work or revising: J.L., J.L., H.B., D.J.L., S.B.L., J.M.L., S.A.J., M.I.K., S.W.Y., M.H.K. Final approval of the manuscript: J.L., J.L., H.B., D.J.L., S.B.L., J.M.L., S.A.J., M.I.K., S.W.Y., M.H.K.
ACKNOWLEDGMENTS
This research was supported by the Basic Research Projects Outstanding Young Scientist Grants through the National Research Foundation (NRF) by the Ministry of Science and ICT (NRF-2022R1C1C1006434).
REFERENCES
3. Lacheta D, Miskiewicz P, Gluszko A, Nowicka G, Struga M, Kantor I, et al. Immunological aspects of Graves’ ophthalmopathy. Biomed Res Int. 2019; 2019:7453260.
4. Smith TJ, Janssen JA. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019; 40:236–67.

5. Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf). 1994; 40:67–72.

6. Chng CL, Lai OF, Chew CS, Peh YP, Fook-Chong SM, Seah LL, et al. Hypoxia increases adipogenesis and affects adipocytokine production in orbital fibroblasts: a possible explanation of the link between smoking and Graves’ ophthalmopathy. Int J Ophthalmol. 2014; 7:403–7.
7. Gortz GE, Horstmann M, Aniol B, Reyes BD, Fandrey J, Eckstein A, et al. Hypoxia-dependent HIF-1 activation impacts on tissue remodeling in Graves’ ophthalmopathy: implications for smoking. J Clin Endocrinol Metab. 2016; 101:4834–42.
9. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013; 93:1–21.

10. Dutton JJ. Anatomic considerations in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018; 34(4S Suppl 1):S7–12.

11. Walasik-Szemplinska D, Pauk-Domanska M, Sanocka U, Sudol-Szopinska I. Doppler imaging of orbital vessels in the assessment of the activity and severity of thyroid-associated orbitopathy. J Ultrason. 2015; 15:388–97.

12. Lee K, Kang JE, Park SK, Jin Y, Chung KS, Kim HM, et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010; 80:982–9.
13. Lee K, Ban HS, Naik R, Hong YS, Son S, Kim BK, et al. Identification of malate dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6 using chemical probes. Angew Chem Int Ed Engl. 2013; 52:10286–9.
14. Kim MH, Lee TH, Lee JS, Lim DJ, Lee PC. Hif-1α inhibitors could successfully inhibit the progression of differentiated thyroid cancer in vitro. Pharmaceuticals (Basel). 2020; 13:208.

17. Liu ZZ, Tian YF, Wu H, Ouyang SY, Kuang WL. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1α/VEGF axis. Neoplasma. 2020; 67:111–8.

18. Befani C, Liakos P. The role of hypoxia-inducible factor-2 alpha in angiogenesis. J Cell Physiol. 2018; 233:9087–98.

19. Luo L, Luo G, Fang Q, Sun Z. Stable expression of hypoxiainducible factor-1α in human renal proximal tubular epithelial cells promotes epithelial to mesenchymal transition. Transplant Proc. 2014; 46:130–4.

20. Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013; 35:965–73.

21. Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, et al. PI3K/ Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review). Mol Med Rep. 2019; 19:783–91.
23. Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun. 2005; 331:718–25.

24. Dong S, Liang S, Cheng Z, Zhang X, Luo L, Li L, et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1αinduced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res. 2022; 41:15.

25. Sharma A, Sinha S, Shrivastava N. Therapeutic targeting hypoxia-inducible factor (HIF-1) in cancer: cutting gordian knot of cancer cell metabolism. Front Genet. 2022; 13:849040.

26. Abdi Sarabi M, Shiri A, Aghapour M, Reichardt C, Brandt S, Mertens PR, et al. Normoxic HIF-1α stabilization caused by local inflammatory factors and its consequences in human coronary artery endothelial cells. Cells. 2022; 11:3878.

27. Ruan H, Zhang Q, Zhang YP, Li SS, Ran X. Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics-a narrative review. Crit Care. 2024; 28:100.

28. Smith TJ, Janssen JA. Building the case for insulin-like growth factor receptor-I involvement in thyroid-associated ophthalmopathy. Front Endocrinol (Lausanne). 2017; 7:167.

29. Gortz GE, Philipp S, Bruderek K, Jesenek C, Horstmann M, Henning Y, et al. Macrophage-orbital fibroblast interaction and hypoxia promote inflammation and adipogenesis in Graves’ orbitopathy. Endocrinology. 2022; 164:bqac203.
30. Van Regemorter E, Joris V, Van Regemorter V, Marique L, Behets C, Lengele B, et al. Downregulation of caveolin-1 and upregulation of deiodinase 3, associated with hypoxia-inducible factor-1α increase, are involved in the oxidative stress of Graves’ orbital adipocytes. Thyroid. 2021; 31:627–37.

31. Gao J, Zhu J, Zhao Y, Gan X, Yu H. Leptin attenuates hypoxia-induced apoptosis in human periodontal ligament cells via the reactive oxygen species-hypoxia-inducible factor-1α pathway. Exp Physiol. 2021; 106:1752–61.

33. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002; 87:2352–8.
34. Westra J, Brouwer E, Bos R, Posthumus MD, Doornbos-van der Meer B, Kallenberg CG, et al. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann N Y Acad Sci. 2007; 1108:340–8.
Fig. 1.
Hypoxia-inducible factor (HIF)-1α expression in orbital fibroblasts. (A) HIF-1α levels in primary cells from thyroid-associated ophthalmopathy (TAO) and non-TAO individuals were assessed under normoxic and hypoxic conditions through Western blot analysis. (B) HIF-1α expression, normalized to β-actin, was quantified in both TAO and non-TAO tissues under normoxic (left) and hypoxic (right) conditions. For each condition, cells under hypoxia were incubated for 48 hours in an environment containing 1% O2, 5% CO2, and 94% N2 at 37°C. For normoxia, cells were incubated for 48 hours in 5% CO2 at 37°C. The samples were taken from TAO patients 112, 144, and 172, and from non-TAO individuals 87, 140, and 143. Data are presented as mean±standard deviation, with n=3 for each group.
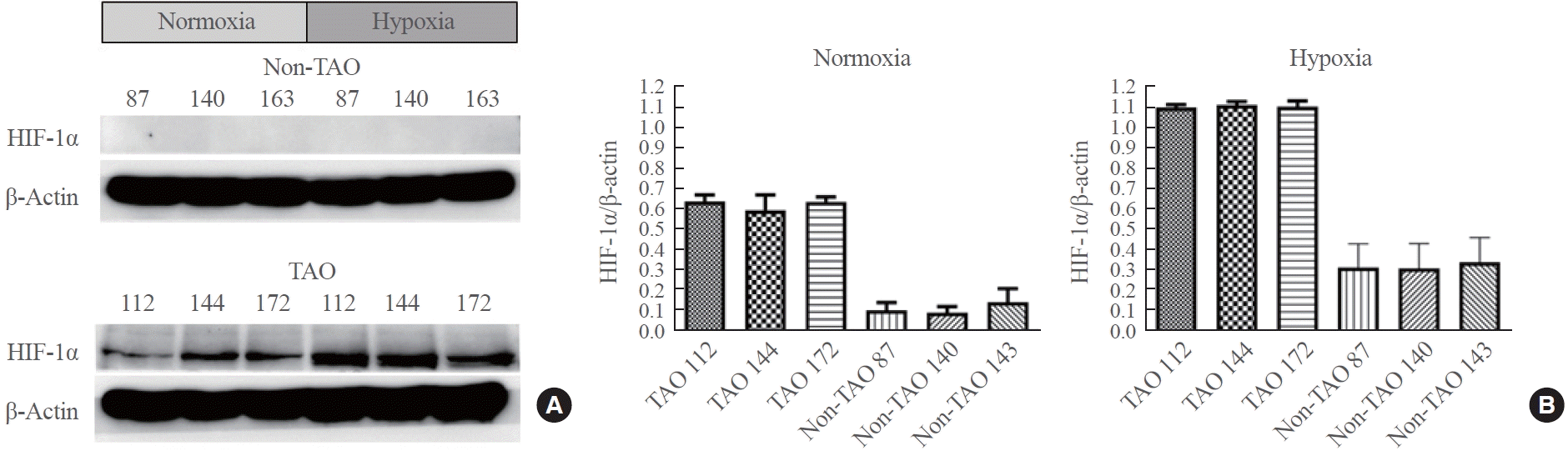
Fig. 2.
Adipogenic changes in orbital fibroblasts derived from patients with thyroid-associated ophthalmopathy (TAO) under hypoxic stimuli. The adipogenic changes in orbital fibroblasts obtained from patients with TAO were prominent under hypoxic conditions. A marked increase in the accumulation of lipid droplets was observed under hypoxia (B) compared to normoxia (A) (upper: 40×; lower: 400×).
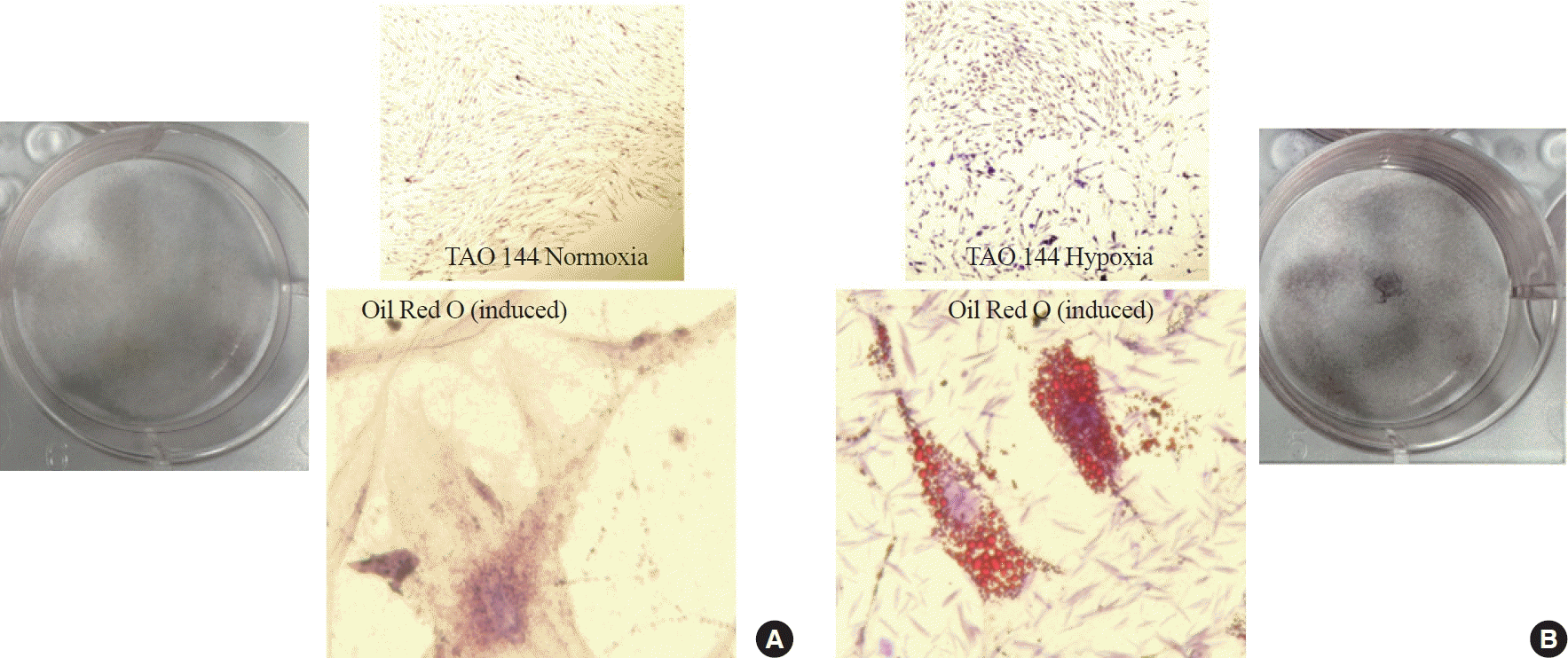
Fig. 3.
Differential expression of adipogenic proteins in thyroid-associated ophthalmopathy (TAO) orbital fibroblasts (OFs) under normoxic and hypoxic conditions. Higher hypoxia-inducible factor (HIF)-1α expression was universally observed in all TAO OFs exposed to hypoxic conditions. (A) The expression of CCAAT/enhancer binding protein (CEBP), and peroxisome proliferator-activated receptor γ (PPARγ), key adipogenic markers, exhibited elevations under hypoxic conditions compared to normoxic conditions. Boxplots display the relative expression levels of HIF-1α, CEBP, and PPARγ across three independent TAO fibroblast samples (TAO 112, TAO 144, and TAO 172). (B) Light gray boxes indicate normoxia, and dark gray boxes indicate hypoxia. The sample size for each condition is n=7. aStatistically significant differences between normoxic and hypoxic conditions within each sample, as determined by the Wilcoxon signed-rank test.
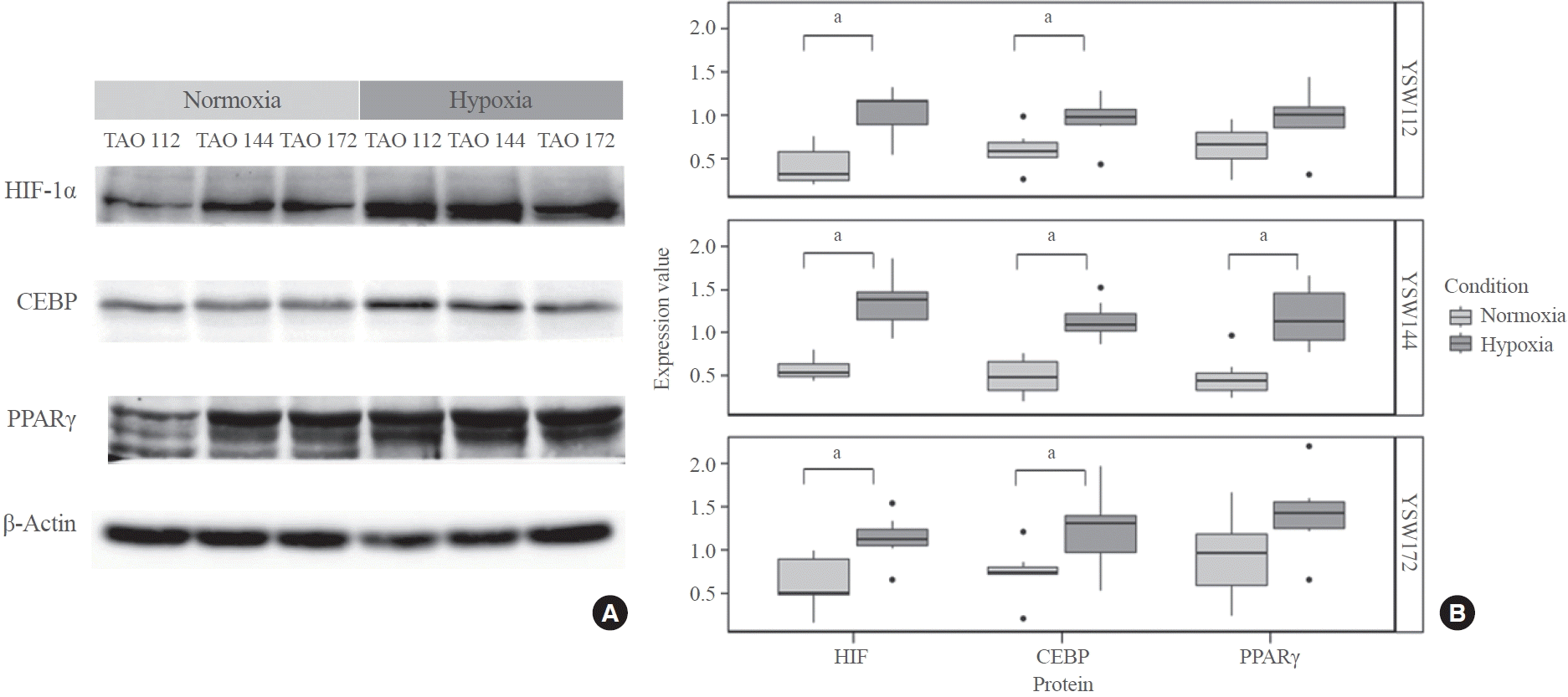
Fig. 4.
Effect of hypoxia-inducible factor (HIF)-1α inhibition on adipogenic protein expression. (A) Inhibition of HIF-1α under hypoxic conditions leads to the modulation of HIF-1α, CCAAT/enhancer binding protein (CEBP), and peroxisome proliferator-activated receptor γ (PPARγ) expression. (B) Boxplots of protein expression levels for HIF-1α, CEBP, and PPARγ under various conditions. The conditions include a control group (hypoxia) and treatments with IDF-11774, a HIF-1α inhibitor, at concentrations of 15 and 30 μM. Statistical analysis was performed using analysis of variance (ANOVA) followed by the Tukey post hoc test to determine significant differences between groups, with each group comprising seven samples. P values are indicated above the brackets connecting the groups being compared. P values less than 0.05 are considered to indicate statistically significant differences between the expression levels of the proteins.
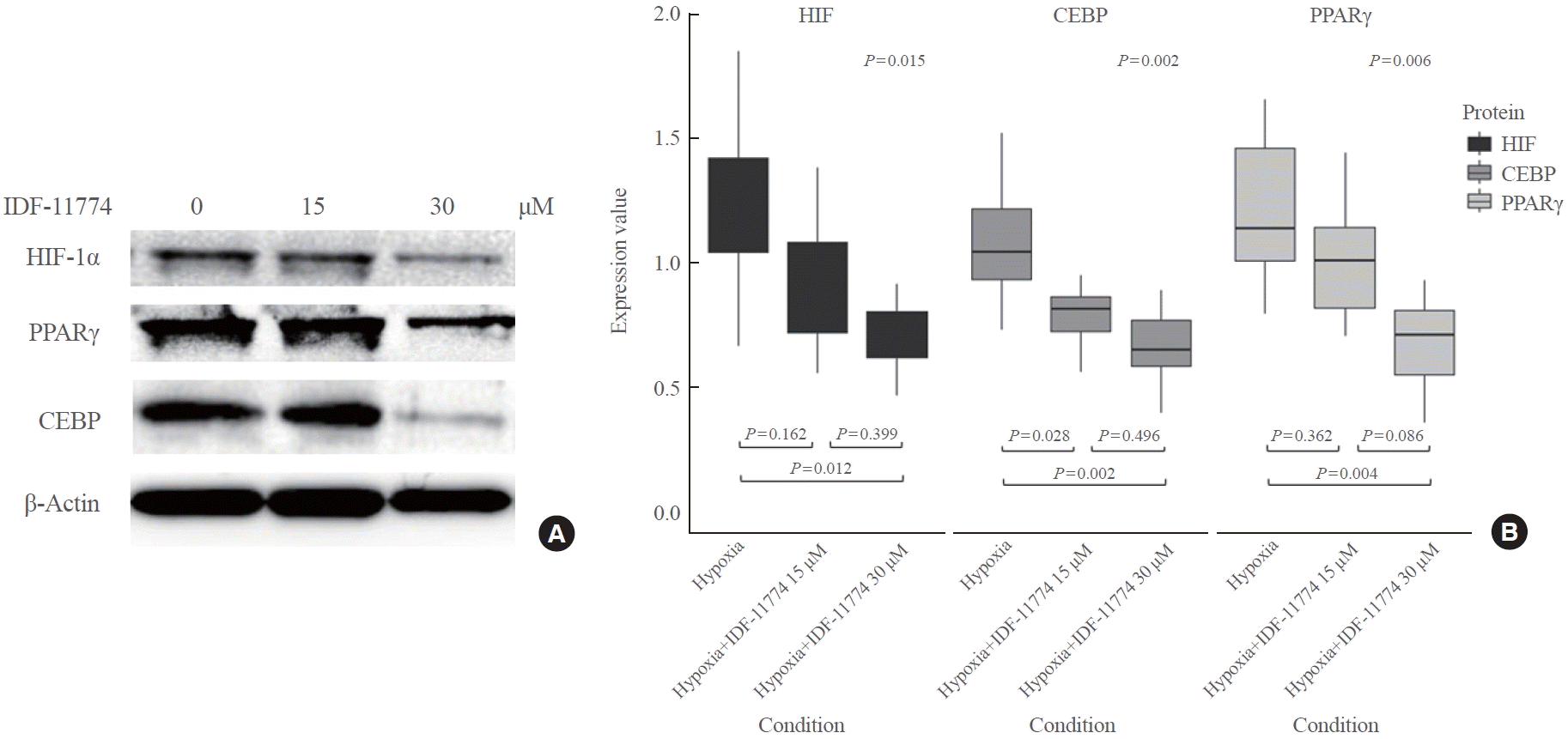
Fig. 5.
Impact of hypoxia-inducible factor (HIF)-1α inhibition on adipogenic protein expression in thyroid-associated ophthalmopathy (TAO) orbital fibroblasts under hypoxia with insulin-like growth factor (IGF)-1 stimulation. (A) The figure shows the expression levels of HIF-1α, CCAAT/enhancer binding protein (CEBP), and peroxisome proliferator-activated receptor γ (PPARγ) in TAO orbital fibroblasts (TAO 144) under hypoxia conditions with IGF-1 (20 ng/mL) stimulation, assessing the impact of HIF-1α inhibition using IDF-11774 at concentrations of 15 and 30 μM. (B) Box plots represent expression values for each protein (HIF-1α, CEBP and PPARγ) across the three conditions: hypoxia alone (black boxes), hypoxia+IDF-11774 15 μM (dark gray boxes), and hypoxia+IDF-11774 30 μM (light gray boxes). The y-axis denotes expression values, and the x-axis indicates treatment conditions. Statistical analysis, including analysis of variance (ANOVA) and post hoc tests, are provided, with P values indicating the significance of differences between groups. Notably, HIF-1α expression decreased in response to IDF-11774 treatment (P=0.062). For CEBP, significant differences were observed between hypoxia and hypoxia+IDF-11774 30 μM (P=0.007), and between IDF-11774 15 and 30 μM (P=0.046). The changes in PPARγ expression were not statistically significant (P=0.553).
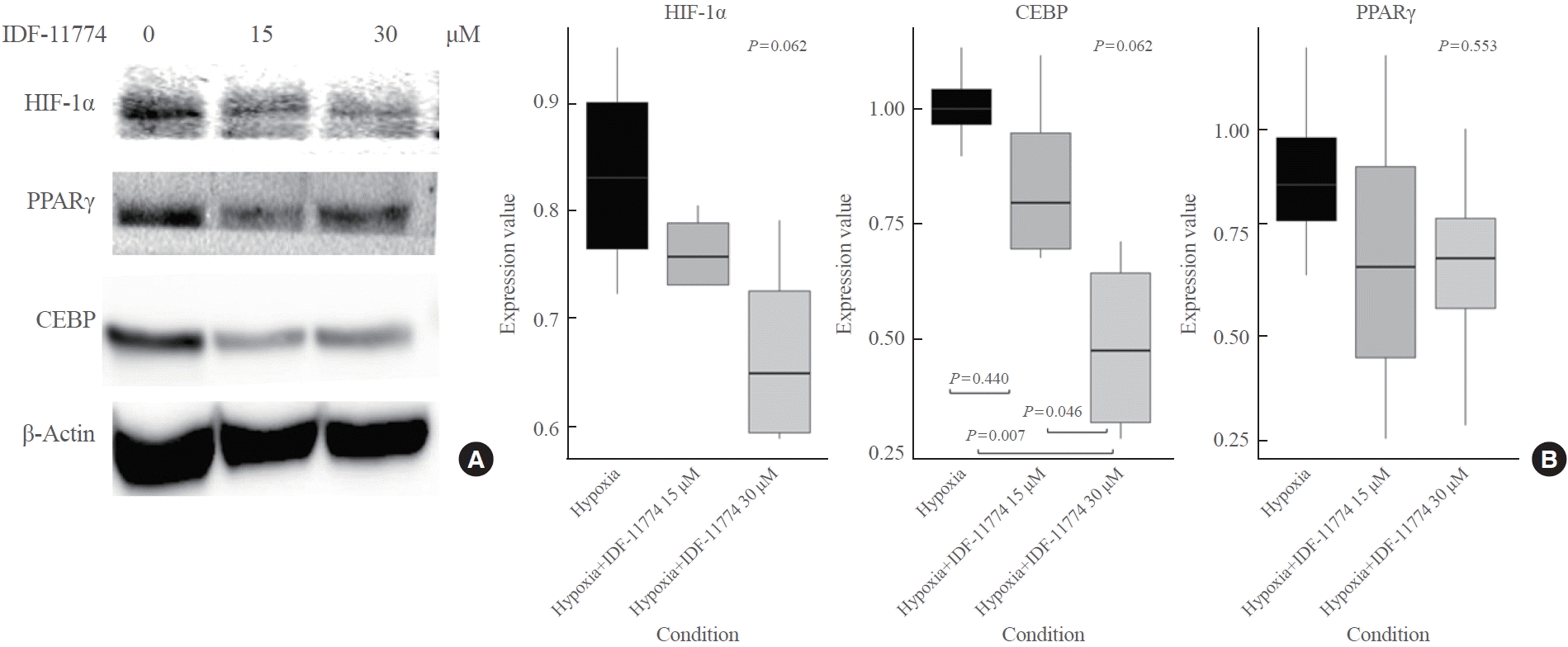
Fig. 6.
Effect of hypoxia-inducible factor (HIF)-1α inhibition on interleukin 6 (IL-6) expression levels in thyroid-associated ophthalmopathy (TAO) orbital fibroblasts under hypoxia with insulin-like growth factor (IGF)-1 stimulation. The box plot presents the effect of HIF-1α inhibition on IL-6 expression levels in TAO orbital fibroblasts (TAO 144) under hypoxic conditions with IGF-1 stimulation (20 ng/mL). The three conditions compared are hypoxia alone, hypoxia with IDF-11774, a HIF-1α inhibitor, at 15 μM, and hypoxia with IDF-11774 at 30 μM. The data indicate that hypoxia significantly increased IL-6 expression. However, the addition of IDF-11774 at both concentrations of 15 and 30 μM resulted in a notable reduction in IL-6 levels. This suggests that HIF-1α plays a critical role in IL-6 expression in TAO orbital fibroblasts under hypoxic conditions, and its inhibition can effectively downregulate IL-6 levels.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download