Abstract
Background and Purpose
Methods
Results
Conclusion
Supplementary materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Figure 1.
Notes
Conflicts of interest
Dr. Regenhardt serves on a DSMB for a trial sponsored by Rapid Medical, serves as site PI for studies sponsored by Penumbra and Microvention, and receives stroke research grant funding from the National Institutes of Health, Society of Vascular and Interventional Neurology, and Heitman Stroke Foundation. Dr. Guenego reports consultancy for Rapid Medical and Phenox, not directly related to the present work. Dr. Clarençon reports conflicts of interest with Medtronic, Balt Extrusion (consultant), ClinSearch (core lab), Penumbra, Stryker (payment for reading) and Artedrone (Board); all not directly related to the present work. Dr. Henninger received support from CDMRP/DoD W81XWH-19-PRARP-RPA and NINDS NS131756, during the conduct of the study. Dr. Liebeskind is consultant as Imaging Core Lab to Cerenovus, Genentech, Medtronic, Stryker, Rapid Medical. Dr. Yeo reports Advisory work for AstraZeneca, Substantial support from NMRC Singapore and is a medical advisor for See-mode, Cortiro and Sunbird Bio, with equity in Ceroflo. All unrelated to the present work. Dr. Griessenauer reports a proctoring agreement with Medtronic and research funding by Penumbra. Dr. Marnat reports conflicts of interest with Microvention Europe, Stryker Neurovascular, Balt (consulting), Medtronic, Johnson & Johnson and Phenox (paid lectures), all not directly related to the present work. Dr. Puri is a consultant for Medtronic Neurovascular, Stryker NeurovascularBalt, Q’Apel Medical, Cerenovus, Microvention, Imperative Care, Agile, Merit, CereVasc and Arsenal Medical, he received research grants from NIH, Microvention, Cerenovus, Medtronic Neurovascular and Stryker Neurovascular, and holds stocks in InNeuroCo, Agile, Perfuze, Galaxy and NTI. Dr. Tjoumakaris is a consultant for Medtronic and Microvention (funds paid to institution, not personally). Dr. Jabbour is a consultant for Medtronic, Microvention and Cerus. All remaining authors have declared no conflicts of interest.
Author contribution
Conceptualization: HAS. Study design: AD, AG. Methodology: HAS, AD, AG. Data collection: HAS, VY, BM, NA, KN, NH, SHS, AK, JK, SG, LS, BT, JH, RR, NC, JB, AR, JF, SS, ME, AP, CD, MC, XB, LR, JF, PH, RR, TM, JS, TO, AM, PJ, AB (Arundhati Biswas), FC, JS, TN, RV, AB (Amanda Baker), DA, NG, MM, VC, BG, CS, MA, CH, HS, DL, AP, AA, IT, TF, EK, BL, AP, VP, AG, AD. Investigation: HAS, VY, BM, NA, KN, NH, SHS, AK, JK, SG, LS, BT, JH, RR, NC, JB, AR, JF, SS, ME, AP, CD, MC, XB, LR, JF, PH, RR, TM, JS, TO, AM, PJ, AB (Arundhati Biswas), FC, JS, TN, RV, AB (Amanda Baker), DA, NG, MM, VC, BG, CS, MA, CH, HS, DL, AP, AA, IT, TF, EK, BL, AP, VP, AG, AD. Statistical analysis: HAS. Writing—original draft: HAS, VY. Writing—review & editing: HAS, VY, BM, NA, KN, NH, SHS, AK, JK, SG, LS, BT, JH, RR, NC, JB, AR, JF, SS, ME, AP, CD, MC, XB, LR, JF, PH, RR, TM, JS, TO, AM, PJ, AB (Arundhati Biswas), FC, JS, TN, RV, AB (Amanda Baker), DA, NG, MM, VC, BG, CS, MA, CH, HS, DL, AP, AA, IT, TF, EK, BL, AP, VP, AG, AD. Approval of final manuscript: all authors.
ACKNOWLEDGMENTS
References
Figure 1.
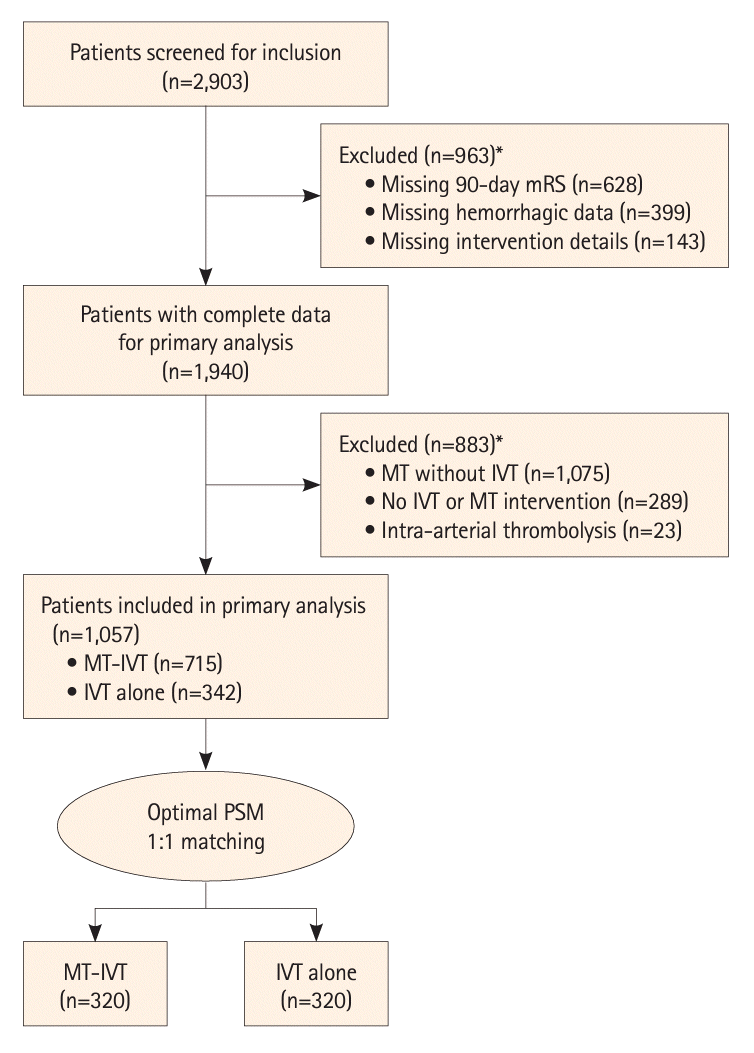
Figure 2.
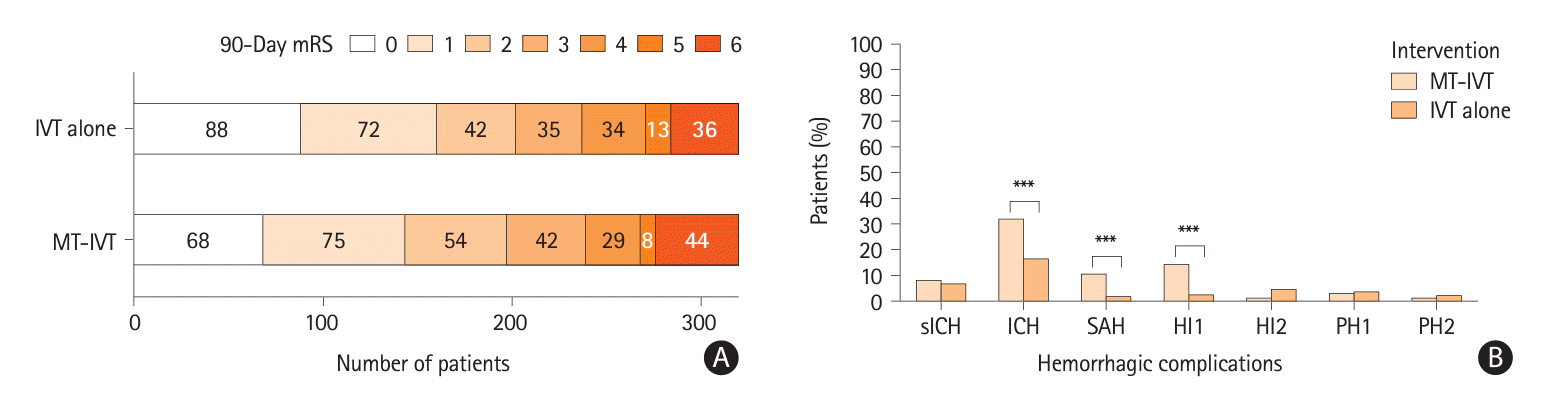
Figure 3.
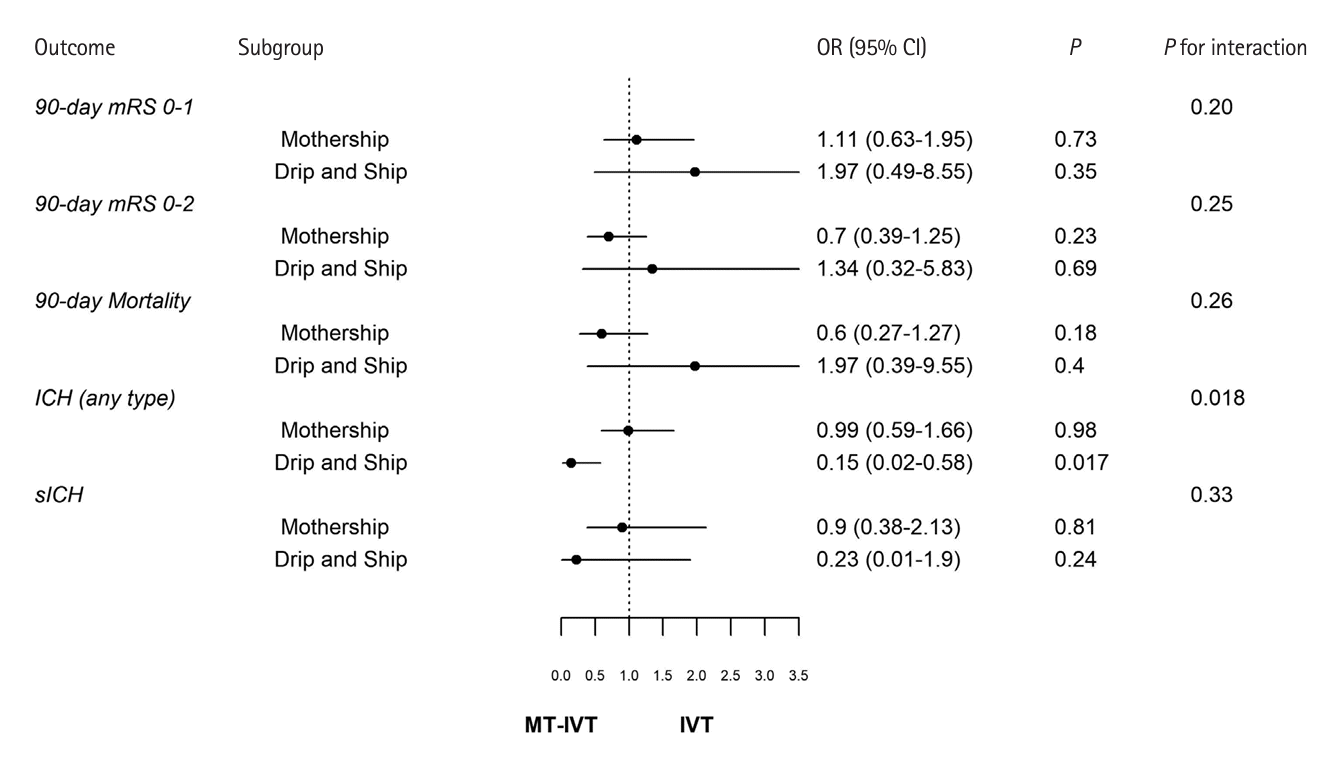
Figure 4.
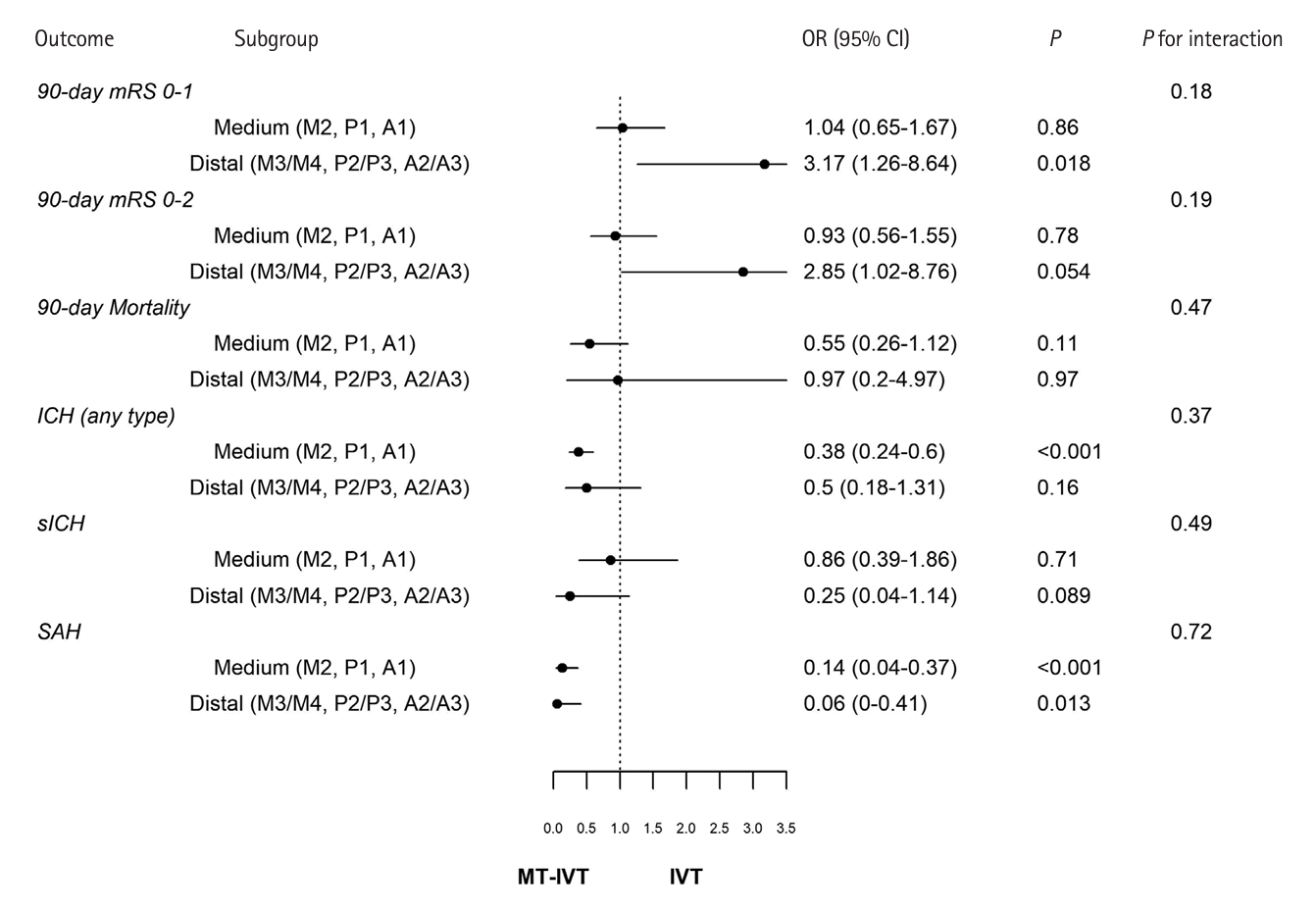
Table 1.
Table 2.
Table 3.
| Variable | Overall (n=640) | MT-IVT (n=320) | IVT alone (n=320) | P |
|---|---|---|---|---|
| Total number of passes | - | 1.00 (1.00–3.00) | NA | |
| Day one NIHSS | 3 (1–8) | 3 (1–11) | 3 (1–6) | 0.061 |
| NIHSS shift | -2 (-5–1) | -2 (-5–2) | -2 (-6–0) | 0.046* |
| mTICI 2c–3 | - | 187 (61) | NA | |
| mTICI 2b–3 | - | 279 (91) | NA | |
| FPE | - | 110 (38) | NA | |
| 90-day mRS 0–1 | 303 (47) | 143 (45) | 160 (50) | 0.21 |
| 90-day mRS 0–2 | 399 (62) | 197 (62) | 202 (63) | 0.74 |
| 90-day mortality | 80 (13) | 44 (14) | 36 (11) | 0.40 |
| sICH | 48 (7.6) | 26 (8.3) | 22 (6.9) | 0.59 |
| ICH (any type) | 155 (24) | 102 (32) | 53 (17) | <0.001*** |
| ICH (by type) | ||||
| HI1 | 54 (8.6) | 46 (1.4) | 8 (2.5) | <0.001*** |
| HI2 | 19 (3.0) | 4 (1.3) | 15 (4.8) | 0.01* |
| PH1 | 22 (3.5) | 10 (3.2) | 12 (3.8) | 0.82 |
| PH2 | 11 (1.7) | 4 (1.3) | 7 (2.2) | 0.54 |
| SAH | 40 (6.3) | 34 (1.1) | 6 (1.9) | <0.001*** |
| Embolization in new territories | 12 (3.5) | 12 (3.8) | 0 (0) | 0.57 |
| Perforation | 14 (4.1) | 14 (4.5) | 0 (0) | 0.48 |
| Artery dissection | 3 (0.9) | 3 (1.0) | 0 (0) | >0.99 |
Values are presented as median (interquartile range) or n (%).
MT, mechanical thrombectomy; IVT, intravenous thrombolysis; NIHSS, National Institutes of Health Stroke Scale; mTICI, modified Thrombolysis in Cerebral Infarction; FPE, first-pass effect; mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage; ICH, intracerebral hemorrhage; HI1, hemorrhagic infarction type 1; HI2, hemorrhagic infarction type 2; PH1, parenchymal hemorrhage type 1; PH2, parenchymal hemorrhage type 2; SAH, subarachnoid hemorrhage; NA, not applicable.
Table 4.
IVT, intravenous thrombolysis; MT, mechanical thrombectomy; OR, odds ratio; CI, confidence interval; mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage; ICH, intracerebral hemorrhage; HI1, hemorrhagic infarction type 1; HI2, hemorrhagic infarction type 2; PH1, parenchymal hemorrhage type 1; PH2, parenchymal hemorrhage type 2; SAH, subarachnoid hemorrhage.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download