Abstract
Background
The use of remdesivir in solid organ transplant recipients (SOTRs) with coronavirus disease 2019 (COVID-19) has been studied. The present systematic review and analysis aimed to assess its effectiveness in this population.
Methods
A comprehensive search of PubMed, Cochrane Library, Web of Science, Embase, medRxiv, and Google Scholar was conducted to identify relevant articles published up to April 2024. The quality of the included studies was evaluated using the Cochrane assessment tool. Data analysis was performed using the Comprehensive Meta-Analysis software ver. 3.0.
Results
The meta-analysis included seven eligible retrospective studies, involving a total of 574 SOTRs. The findings indicated no significant differences in mortality rate (odds ratio [OR], 1.19; 95% confidence interval [CI], 0.59–2.39), hospitalization rate (OR, 0.69; 95% CI, 0.10–4.79), need for mechanical ventilation (OR, 0.98; 95% CI, 0.44–2.18), or need for oxygen therapy (OR, 3.73; 95% CI, 0.75–18.34) between the groups that received remdesivir and those that did not. However, a statistically significant difference was observed in the rate of intensive care unit admissions between the two groups (OR, 2.39; 95% CI, 1.24–4.57).
Solid organ transplant recipients (SOTRs) are significantly impacted by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the primary cause of coronavirus disease 2019 (COVID-19) [1]. Studies using real-world data have shown that SOTRs infected with SARS-CoV-2 face a higher mortality risk than the general population [2,3]. Furthermore, SOTRs are more prone to severe illness from COVID-19, leading to increased rates of hospitalization and intensive care unit (ICU) admissions compared to the general population [3,4]. This heightened vulnerability to severe COVID-19 among SOTRs can largely be attributed to their ongoing immunosuppression, older age, presence of underlying health conditions, and frequent exposure to healthcare settings [5]. Despite the widespread distribution of numerous vaccine doses, which may mitigate the impact of this highly transmissible variant, the severity, thromboembolic complications, mortality rates, and hospitalization rates remain higher than those seen in the general population [6,7]. This underscores the urgent need for innovative therapeutic and preventative strategies to tackle the persistent challenges posed by the virus [6,8]. Evidence indicates that while booster vaccinations improve the immune response to COVID-19 vaccines in SOTRs, a significant number still do not develop detectable humoral immunity after the third dose [9]. Given the reduced effectiveness of COVID-19 vaccines in SOTRs compared to non-SOTRs, it is crucial to consider additional protective measures alongside vaccination [10]. Various therapeutic agents, including nirmatrelvir/ritonavir (Paxlovid), molnupiravir, sotrovimab, and remdesivir, have been evaluated and suggested for treating SOTRs infected with COVID-19 [11–14]. Remdesivir, in particular, is a versatile antiviral medication effective against viruses from different families, including coronaviruses. It acts as a nucleotide prodrug, and its active metabolite inhibits viral RNA-dependent RNA polymerases, essential enzymes in the replication of a wide range of viruses, including those from the Coronaviridae family [15].
Prior to our study, numerous real-world studies have been conducted on the use of remdesivir in SOTRs with SARS-CoV-2 infection [16–19]. Currently, there is no comprehensive systematic review and meta-analysis available that assesses the effectiveness of remdesivir specifically in SOTRs with SARS-CoV-2 infection.
We registered the protocol for this systematic review and meta-analysis under the registry number CRD42024556446, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [20] (Supplementary Material 1).
PubMed, Cochrane Library, Web of Science, Embase, medRxiv, and Google Scholar were independently searched by two authors to identify studies published up to April 2024, using a search strategy specifically designed for this study. Additionally, the reference lists of the retrieved articles were scanned for further relevant studies. The searches were restricted to publications in English. The search terms used included “2019-novel coronavirus,” “SARS-CoV-2,” “COVID-19,” “2019-nCoV,” and “remdesivir.” The detailed search strategy for each database is provided in Supplementary Material 2.
Two authors independently screened and reviewed the titles and abstracts of publications identified in the databases, using the predefined inclusion and exclusion criteria. This initial screening for potential eligibility was followed by a full-text assessment of the articles.
Inclusion criteria
(1) Population: the analysis included studies that specifically focused on SOTRs to ensure that the findings would be directly applicable to this vulnerable population. (2) Intervention: we included studies that investigated the use of remdesivir as a treatment option, allowing us to assess its efficacy in SOTRs. (3) Control: the analysis included studies that compared remdesivir to any therapeutic intervention, standard of care (SOC), or placebo, which helped us evaluate its relative effectiveness. (4) Outcomes: the studies were required to report key clinical outcomes, including mortality rate, hospitalization rate, need for mechanical ventilation, oxygen therapy, and ICU admission.
Exclusion criteria
(1) Irrelevance to the research aims: studies that did not directly address the impact of remdesivir on SOTRs were excluded to maintain focus on our research question. (2) Lack of relevant data: we excluded studies that did not provide sufficient data on the outcomes of interest, ensuring that only studies with robust findings were included. (3) Study type: we excluded animal models, in vitro or in vivo studies, case reports, letters to editors, and editorials, as these did not provide the level of evidence required for our analysis.
We evaluated the methodological quality of nonrandomized intervention studies using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [21]. This comprehensive tool assesses various biases, including those associated with confounding factors, participant selection, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and the selection of reported results. To evaluate the certainty of the evidence, we employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool, which categorizes the strength of evidence into four levels: very low, low, moderate, and high [22].
Two authors independently extracted data from published summary estimate data. Any discrepancies between the reviewers were resolved either by reaching a consensus or by involving a third author. The data extracted from each eligible study included details such as the author’s name, year of publication, country of origin, sample size, average age of participants, treatment duration, and the outcomes of effectiveness. This meticulous approach ensured the accuracy and completeness of the information gathered for our analysis.
We used the Comprehensive Meta-Analysis software ver. 3.0 (Biostat) to compare the effectiveness of remdesivir with that of the control group. For dichotomous variables, the data were analyzed using the odds ratio (OR) with a 95% confidence interval (CI). High heterogeneity was defined as I2>50% and P<0.1. Under these conditions, random-effect models were applied, whereas fixed-effect models were used when heterogeneity was low. Subgroup analyses were conducted based on SARS-CoV-2 variants and the types of treatments used in the control group. Additionally, a sensitivity analysis was performed using the leave-one-out method to assess the robustness of the results. The ORs were also recalculated by reversing the comparison to more accurately reflect the direction of the effect (Supplementary Figs. 1–5).
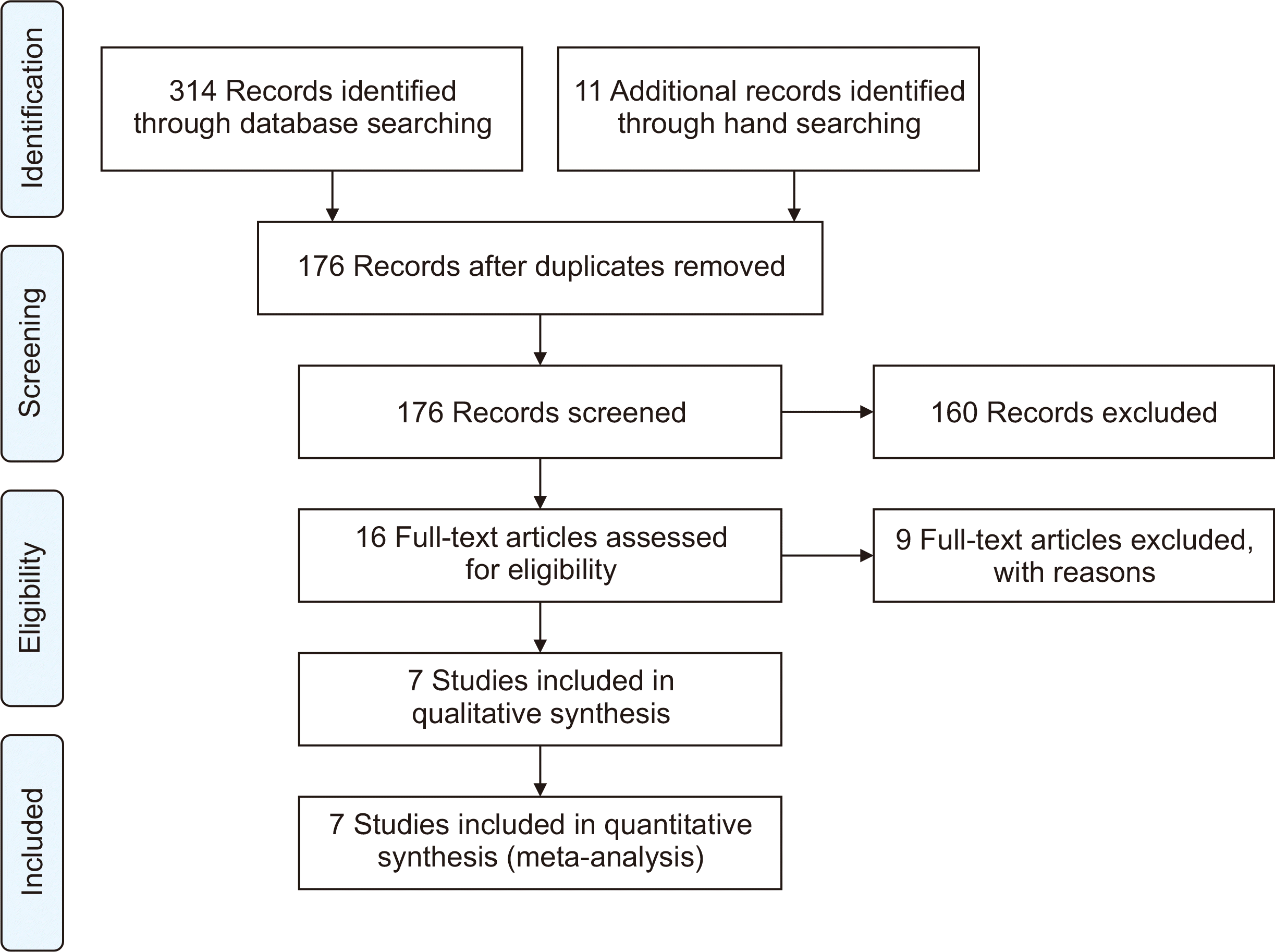
The flow diagram that illustrates the process of identifying, screening, and selecting studies based on their titles, abstracts, and full texts is presented in Fig. 1. Initially, 325 studies were identified. After removing duplicates, 176 studies were reviewed based on their titles and abstracts. Of these, 160 studies were excluded based on the predefined inclusion criteria. The full texts of the remaining 16 studies were thoroughly reviewed, resulting in the inclusion of seven studies [16–19,23–25]. All included studies were observational and involved a total of 574 SOTRs who met the inclusion criteria. The data collection period for the studies spanned from 2021 to 2022. In two studies, remdesivir was compared to molnupiravir, while in the remaining studies, remdesivir was compared to the standard of care. The key characteristics of the included studies are summarized in Table 1.
The risk of bias assessments using the ROBINS-I tool revealed varying levels of concern across the included studies, with most exhibiting serious risk in the confounding and selection domains. Studies with a serious risk of bias in confounding may have significant issues related to participant selection and the management of confounding factors, potentially affecting the validity of their findings. All studies received a moderate rating for the classification of interventions, deviations from intended interventions, measurement of outcomes, and reported results. The assessment of low risk for missing data across all studies indicates that data completeness was generally well managed, with minimal concerns about the impact of missing data on the study outcomes. The detailed assessment results for each study's risk of bias are provided in Supplementary Table 1. The certainty assessment of the evidence regarding the effectiveness of remdesivir in various clinical outcomes indicates a low level of certainty across all evaluated outcomes. These findings highlight the necessity for a cautious interpretation of remdesivir's effectiveness, as the low certainty across all outcomes underscores the need for further research to clarify its role in treating SOTRs with SARS-CoV-2 infection. The certainty of evidence for all key outcomes is presented in Supplementary Table 2.
Mortality rate
Five studies [16,18,23–25] with a total population of 549 patients, reported on mortality rates. The pooled estimate indicated no significant difference in the mortality rates between the remdesivir and control groups (OR, 1.19; 95% CI, 0.59–2.39; P=0.61; I2=90%) (Fig. 2). This result suggests that remdesivir may not offer a significant benefit to SOTRs with COVID-19 compared to other treatment approaches or SOC.
Hospitalization rate
Five studies [16,18,19,23,25] with a total population of 384 patients reported the hospitalization rate in SOTRs with COVID-19. The pooled analysis revealed no significant difference in the hospitalization rates between the remdesivir and control groups (OR, 0.69; 95% CI, 0.10–4.79; P=0.71; I2=90%) (Fig. 3). This result indicates that remdesivir treatment does not significantly affect the likelihood of hospitalization for SOTRs infected with COVID-19 compared to standard of care or other therapeutic interventions.
Intensive care unit admission
Four studies [18,23–25] involving 451 patients reported ICU admissions for patients receiving remdesivir and those in control groups. The pooled analysis indicated a significant difference in ICU admissions between SOTRs who received remdesivir and those in the control groups (OR, 2.39; 95% CI, 1.24–4.57; P<0.01; I2=90%) (Fig. 4). This result suggests that the use of remdesivir may be linked to an increased risk of severe disease requiring intensive care among SOTRs.
Need for mechanical ventilation
Three studies [18,24,25] with a total population of 411 patients presented data on the need for mechanical ventilation. The pooled analysis indicated no significant difference in the requirement for mechanical ventilation between the remdesivir and control groups (OR, 0.98; 95% CI, 0.44–2.18; P=0.97; I2=78%) (Fig. 5). This suggests that the use of remdesivir does not significantly alter the likelihood of requiring mechanical ventilation in SOTRs with COVID-19 compared to SOC or other therapeutic interventions.
Need for oxygen therapy
In four studies [16–18,24] with a total of 480 COVID-19 patients, the need for oxygen therapy was reported. The pooled analysis revealed no significant difference in the need for mechanical ventilation between the remdesivir and control groups (OR, 3.73; 95% CI, 0.75–18.34; P=0.10; I2=90%) (Supplementary Fig. 6). The P-value of 0.10 further suggests that the difference is not statistically significant, indicating that remdesivir may not significantly affect the need for mechanical ventilation in this patient population.
Sensitivity analysis and subgroup analysis
The results of subgroup analyses by control group and type of SARS-CoV-2 variant are presented in Supplementary Table 3. The leave-one-out sensitivity analysis showed no significant changes in the effect estimate for outcomes such as mortality rate, hospitalization rate, ICU admission, the need for mechanical ventilation, and the need for oxygen therapy, as detailed in Supplementary Figs. 7–11. However, this analysis indicated that the pooled estimate for ICU admission was not robust. Excluding certain studies altered the significance of the results, suggesting that the overall findings were sensitive to specific studies.
Given the limited effectiveness of COVID-19 vaccines in SOTRs compared to the general population, along with the increased risk of mortality and morbidity in this group, it is crucial to explore additional treatment options for COVID-19 in SOTRs [26]. One potential treatment under consideration for COVID-19 is the antiviral drug remdesivir. This study aimed to evaluate the potential benefits of remdesivir in treating SOTRs with COVID-19.
The results of the meta-analysis indicated that the use of remdesivir in SOTRs with SARS-CoV-2 infection did not demonstrate any significant clinical benefit in reducing COVID-19-related deaths compared to the control group. Possible explanations for this finding could include high heterogeneity among the included studies, such as variations in treatment protocols, follow-up duration, and the severity of COVID-19 at baseline. Additionally, the increased vulnerability of SOTRs to severe COVID-19 and the timing of remdesivir administration may have contributed to the lack of significant clinical benefit in reducing COVID-19-related deaths in this population [27]. Evidence on the benefit of remdesivir in treating COVID-19 is conflicting. Some meta-analyses suggest its efficacy in reducing death in COVID-19 patients, while others found no benefit [14,28]. Although the current meta-analysis did not demonstrate a clear advantage of using remdesivir in treating SOTRs with COVID-19, evidence does support the effectiveness of monoclonal antibody therapy in improving mortality rates in this patient population [29]. Research by Amani et al. [29] highlighted the effectiveness of sotrovimab monoclonal antibody in reducing mortality rates among SOTRs infected with SARS-CoV-2. Conversely, the evidence supporting antiviral agents like nirmatrelvir/ritonavir and molnupiravir in SOTRs is currently limited [30,31]. Due to the potential for adaptive mutations in spike protein variants that could impact the efficacy of monoclonal antibodies, antiviral agents may be considered a more valuable treatment option [23].
The meta-analysis of studies revealed no significant difference in hospitalization rates between SOTRs infected with SARS-CoV-2 who were treated with remdesivir and those who were not. Remdesivir is typically administered in hospital settings, often after the disease has progressed, which might explain the timing of its administration in SOTRs with SARS-CoV-2. However, several individual studies have indicated that remdesivir could effectively reduce the risk of hospitalization in outpatients with COVID-19 [32,33]. For example, Gottlieb et al. [32] demonstrated that a 3-day course of early remdesivir effectively prevented COVID-19-related hospitalizations in outpatients with mild to moderate disease. Similarly, Mazzitelli et al. [33] found that early treatment with remdesivir reduced the risk of hospitalization compared to controls. In studies involving SOTRs infected with SARS-CoV-2 and treated with remdesivir, the findings were inconsistent [16,19,23]. Colaneri et al. [23] reported a significantly lower rate of hospital admission or worsening of COVID-19 in SOTRs who received remdesivir compared to those who did not (0% vs. 52.9%). Conversely, Villamarín et al. [19] observed a lower percentage of progression to pneumonia and hospital admission in kidney transplant recipients with COVID-19 who were treated with remdesivir compared to those treated with molnupiravir. Cacho et al. [16] noted that the discharge rate was 84% for SOTRs receiving remdesivir, compared to 92.9% for those not receiving it.
The meta-analysis revealed that SOTRs infected with SARS-CoV-2 who received remdesivir had a higher rate of admission to the ICU compared to those who did not receive the medication. However, a subgroup analysis based on the type of treatment in the control group showed that this difference was not significant. The lack of clinical benefit from remdesivir in reducing ICU admissions may be attributed to high heterogeneity among the studies included in this meta-analysis. Additionally, the timing of remdesivir administration suggests that late treatment could lead to higher rates of severe outcomes, including ICU admissions [34]. However, further sensitivity analysis using the leave-one-out method indicated that this finding might not be robust. In a real-world study, early administration of remdesivir in SOTRs with COVID-19 was associated with a lower percentage of ICU admissions compared to untreated individuals, although this difference was not statistically significant [23]. Additionally, Solera et al. [18] reported that SOTRs treated with remdesivir for SARS-CoV-2 infection did not require ICU admission, while 3% of untreated SOTRs were admitted to the ICU. These results underscore the need for additional research to determine the impact of remdesivir on ICU admissions in SOTRs with COVID-19. The discrepancies between studies highlight the complexity of managing severe cases of COVID-19 in transplant recipients and emphasize the importance of individualized treatment approaches based on patient factors. Further investigation is warranted to better understand the efficacy of remdesivir in preventing ICU admissions and improving outcomes in this vulnerable population.
Based on pooled estimates from various studies, remdesivir did not significantly reduce the need for mechanical ventilation or oxygen therapy in SOTRs with SARS-CoV-2 infection compared to those who did not receive the medication. Remdesivir is typically administered in hospital settings, often after the disease has progressed, potentially increasing the need for mechanical ventilation or oxygen therapy. However, a meta-analysis of clinical trials indicated that the odds of requiring mechanical ventilation or extracorporeal membrane oxygenation were significantly lower in the remdesivir group than in the control group [35]. Studies have also shown that SOTRs infected with SARS-CoV-2 were less likely to need mechanical ventilation when treated with remdesivir than those who were not treated [18,24]. For example, Solera et al. [18] observed no instances of mechanical ventilation in SOTRs treated with remdesivir, while some untreated individuals required ventilation. Similarly, Elec et al. [24] reported that a lower percentage of SOTRs needed mechanical ventilation in the remdesivir group than in those who did not receive the treatment. The variation in results across different studies suggests the need for personalized treatment approaches based on individual patient characteristics to optimize outcomes in this vulnerable population.
Our study has some important limitations that should be acknowledged. First, the inclusion of retrospective studies in this meta-analysis may introduce a significant risk of bias in the results. Furthermore, these studies did not employ matching methods to reduce selection bias, potentially affecting the reliability of our findings. Second, variations in factors such as the rate of COVID-19 vaccination, the severity of COVID-19 at baseline, follow-up duration, type of transplant, control treatments, and treatment protocols across the patient populations could hinder an accurate assessment of the effectiveness of remdesivir. Lastly, the inability to conduct subgroup analyses based on variables like vaccination rate, COVID-19 severity, and transplant type may restrict our ability to accurately estimate the effectiveness of remdesivir in SOTRs with COVID-19.
In conclusion, our meta-analysis reveals that remdesivir does not confer clinical benefits for SOTRs infected with SARS-CoV-2. Several factors necessitate a cautious interpretation of these findings. Firstly, the evidence supporting these conclusions is rated as low in certainty. Secondly, the high risk of bias in the included studies underscores the importance of careful consideration. Given these limitations, clinicians should carefully evaluate the potential benefits and risks of remdesivir, considering each patient's unique circumstances and the severity of their disease. Future research should aim to overcome these limitations by conducting large, well-designed randomized controlled trials or prospective studies that employ matching methods and subgroup analyses based on transplant type and COVID-19 severity to better assess the effectiveness of remdesivir in SOTRs infected with SARS-CoV-2.
Supplementary materials can be found via https://doi.org/10.4285/ctr.24.0031.
ARTICLE INFORMATION
Author Contributions
Conceptualization: ZN, SK. Data curation: AO, EF, ESM. Formal analysis: ZN, SK. Investigation: SHPM, MMI, SFG. Project administration: ZN, SK. Validation: AO, ZN, SHPM. Writing–original draft: SK. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Danziger-Isakov L, Blumberg EA, Manuel O, Sester M. 2021; Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 21:925–37. DOI: 10.1111/ajt.16449. PMID: 33319449. PMCID: PMC9800718.
2. Clarke JA, Wiemken TL, Korenblat KM. 2022; Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic. Transplantation. 106:2399–407. DOI: 10.1097/TP.0000000000004341. PMID: 36042551. PMCID: PMC9696767.
3. Søfteland JM, Li H, Magnusson JM, Leach S, Friman V, Gisslén M, et al. 2024; COVID-19 outcomes and vaccinations in Swedish solid organ transplant recipients 2020-2021: a nationwide multi-register comparative cohort study. Viruses. 16:271. DOI: 10.3390/v16020271. PMID: 38400046. PMCID: PMC10893154.
4. Ryu TH, Kim HY, Ahn J, Oh JS, Kim JK. 2023; Delayed exacerbation of COVID-19 pneumonia in vaccinated kidney transplant recipients receiving immunosuppressants: a case series. Korean J Transplant. 37:63–8. DOI: 10.4285/kjt.22.0043. PMID: 37064773. PMCID: PMC10090830.
5. Mahalingasivam V, Craik A, Tomlinson LA, Ge L, Hou L, Wang Q, et al. 2021; A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 6:24–45. DOI: 10.1016/j.ekir.2020.10.023. PMID: 33163708. PMCID: PMC7607258.
6. Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. 2022; Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 36:e14492. DOI: 10.1111/ctr.14492. PMID: 34558116. PMCID: PMC8646895.
7. Villanego F, Vigara LA, Alonso M, Orellana C, Gómez AM, Eady M, et al. 2022; Trends in COVID-19 outcomes in kidney transplant recipients during the period of omicron variant predominance. Transplantation. 106:e304–5. DOI: 10.1097/TP.0000000000004126. PMID: 35389374. PMCID: PMC9128401.
8. Amani B, Zareei S, Amani B, Zareei M, Zareei N, Shabestan R, et al. 2022; Artesunate, imatinib, and infliximab in COVID-19: a rapid review and meta-analysis of current evidence. Immun Inflamm Dis. 10:e628. DOI: 10.1002/iid3.628. PMID: 35634954. PMCID: PMC9092000.
9. Chen X, Luo D, Mei B, Du J, Liu X, Xie H, et al. 2023; Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis. Clin Microbiol Infect. 29:441–56. DOI: 10.1016/j.cmi.2022.12.004. PMID: 36509376. PMCID: PMC9733302.
10. Huh K, Kang M, Kim YE, Choi Y, An SJ, Seong J, et al. 2024; Risk of severe COVID-19 and protective effectiveness of vaccination among solid organ transplant recipients. J Infect Dis. 229:1026–34. DOI: 10.1093/infdis/jiad501. PMID: 38097377.
11. Gao Y, Liu M, Li Z, Xu J, Zhang J, Tian J. 2023; Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin Microbiol Infect. 29:979–99. DOI: 10.1016/j.cmi.2023.04.014. PMID: 37084941. PMCID: PMC10116122.
12. Amani B, Amani B. 2024; Comparison of effectiveness and safety of nirmatrelvir/ritonavir versus sotrovimab for COVID-19: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 1–9. DOI: 10.1080/14787210.2024.2326561. PMID: 38457124.
13. Amani B, Akbarzadeh A, Amani B, Shabestan R, Khorramnia S, Navidi Z, et al. 2023; Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis. J Med Virol. 95:e28889. DOI: 10.1002/jmv.28889. PMID: 37368841.
14. Angamo MT, Mohammed MA, Peterson GM. 2022; Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 50:27–41. DOI: 10.1007/s15010-021-01671-0. PMID: 34331674. PMCID: PMC8325414.
15. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. 2020; Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 585:273–6. DOI: 10.1038/s41586-020-2423-5. PMID: 32516797. PMCID: PMC7486271.
16. Cacho J, Nicolás D, Bodro M, Cuadrado-Payán E, Torres-Jaramillo V, Gonzalez-Rojas Á, et al. 2022; Use of remdesivir in kidney transplant recipients with SARS-CoV-2 Omicron infection. Kidney Int. 102:917–21. DOI: 10.1016/j.kint.2022.08.001. PMID: 35964801. PMCID: PMC9367173.
17. Fesu D, Bohacs A, Hidvegi E, Matics Z, Polivka L, Horvath P, et al. 2022; Remdesivir in solid organ recipients for COVID-19 pneumonia. Transplant Proc. 54:2567–9. DOI: 10.1016/j.transproceed.2022.10.043. PMID: 36400587. PMCID: PMC9626440.
18. Solera JT, Árbol BG, Bahinskaya I, Marks N, Humar A, Kumar D. 2023; Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave. Am J Transplant. 23:78–83. DOI: 10.1111/ajt.17199. PMID: 36148607. PMCID: PMC9538117.
19. Villamarín M, Márquez-Algaba E, Esperalba J, Perelló M, Los Arcos I, Company D, et al. 2022; Preliminary clinical experience of molnupiravir to prevent progression of COVID-19 in kidney transplant recipients. Transplantation. 106:2200–4. DOI: 10.1097/TP.0000000000004306. PMID: 35915545.
20. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. 2009; Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151:264–9. DOI: 10.7326/0003-4819-151-4-200908180-00135. PMID: 19622511.
21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.
22. Zhang Y, Coello PA, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. 2019; GRADE guidelines: 20. assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J Clin Epidemiol. 111:83–93. DOI: 10.1016/j.jclinepi.2018.05.011. PMID: 29800687.
23. Colaneri M, Amarasinghe N, Rezzonico L, Pieri TC, Segalini E, Sambo M, et al. 2022; Early remdesivir to prevent severe COVID-19 in recipients of solid organ transplant: a real-life study from Northern Italy. Int J Infect Dis. 121:157–60. DOI: 10.1016/j.ijid.2022.05.001. PMID: 35533831. PMCID: PMC9076039.
24. Elec F, Magnusson J, Elec A, Muntean A, Antal O, Moisoiu T, et al. 2022; COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study. Int J Infect Dis. 118:247–53. DOI: 10.1016/j.ijid.2022.03.015. PMID: 35301103. PMCID: PMC8920078.
25. Razia D, Sindu D, Grief K, Cherrier L, Omar A, Walia R, et al. 2023; Molnupiravir vs remdesivir for treatment of Covid-19 in lung transplant recipients. J Heart Lung Transplant. 42:S165–6. DOI: 10.1016/j.healun.2023.02.1653.
26. Khorramnia S, Navidi Z, Orandi A, Iravani MM, Orandi A, Malekabad ES, et al. 2024; Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis. Clin Transplant Res. 38:136–44. DOI: 10.4285/ctr.24.0015. PMID: 38904088. PMCID: PMC11228381.
27. Wong CK, Lau KT, Au IC, Xiong X, Chung MS, Lau EH, et al. 2022; Optimal timing of remdesivir initiation in hospitalized patients with coronavirus disease 2019 (COVID-19) administered with dexamethasone. Clin Infect Dis. 75:e499–508. DOI: 10.1093/cid/ciab728. PMID: 34420051. PMCID: PMC8513400.
28. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. 2021; Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 31:e2187. DOI: 10.1002/rmv.2187. PMID: 33128490.
29. Amani B, Shabestan R, Rajabkhah K, Amani B. 2023; Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis. Korean J Transplant. 37:277–85. DOI: 10.4285/kjt.23.0038. PMID: 37916433. PMCID: PMC10772269.
30. Dhand A, Okumura K, Ohira S, Kapur R, Wolfe K, Nishida S. 2023; Molnupiravir for treatment of COVID-19 in solid organ transplant recipients. Transplantation. 107:e182–3. DOI: 10.1097/TP.0000000000004588. PMID: 36959161. PMCID: PMC10205063.
31. Tang Y, Li Y, Song T. 2023; Optimizing the use of nirmatrelvir/ritonavir in solid organ transplant recipients with COVID-19: a review of immunosuppressant adjustment strategies. Front Immunol. 14:1150341. DOI: 10.3389/fimmu.2023.1150341. PMID: 37081880. PMCID: PMC10111375.
32. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. 2022; Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 386:305–15. DOI: 10.1056/NEJMoa2116846. PMID: 34937145. PMCID: PMC8757570.
33. Mazzitelli M, Trunfio M, Sasset L, Scaglione V, Ferrari A, Mengato D, et al. 2023; Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study. J Med Virol. 95:e28660. DOI: 10.1002/jmv.28660. PMID: 36905216.
34. Alonso-Navarro R, Ramírez M, Masiá M, Paredes R, Montejano R, Povar-Echeverria M, et al. 2023; Time from symptoms onset to remdesivir is associated with the risk of ICU admission: a multicentric analyses. BMC Infect Dis. 23:286. DOI: 10.1186/s12879-023-08222-y. PMID: 37142994. PMCID: PMC10157565.
35. Reddy Vegivinti CT, Pederson JM, Saravu K, Gupta N, Barrett A, Davis AR, et al. 2021; Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 62:43–8. DOI: 10.1016/j.amsu.2020.12.051. PMID: 33489115. PMCID: PMC7806502.
Fig. 2
Forest plot of mortality rates comparing remdesivir to the control group in coronavirus disease 2019 patients. The forest plot indicates no significant difference in mortality rates between the remdesivir and control groups. CI, confidence interval.
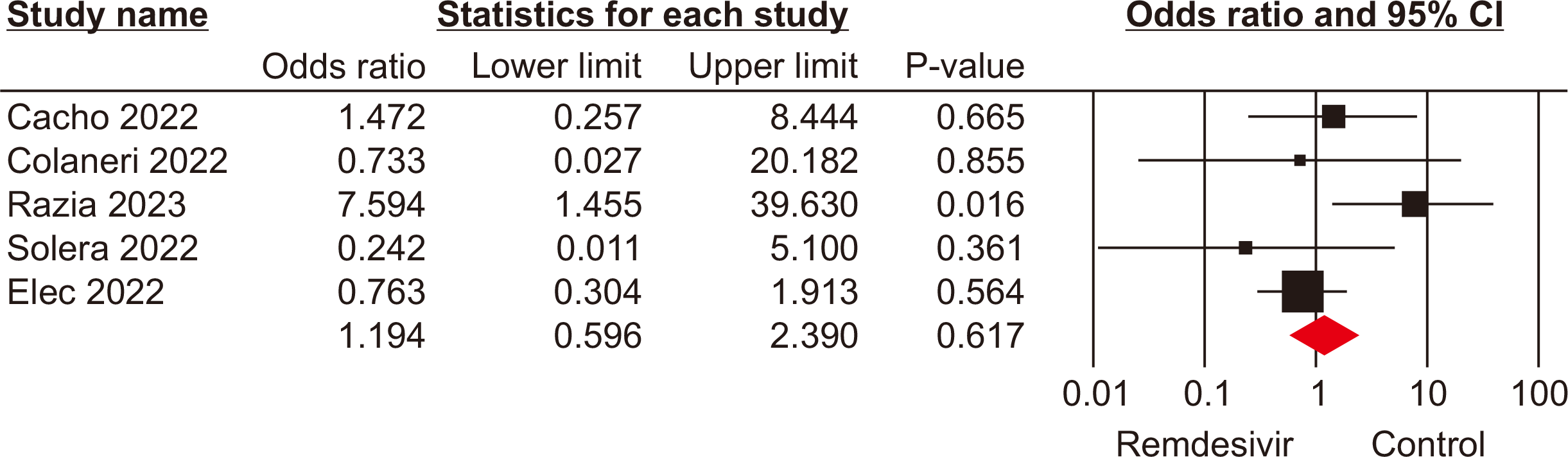
Fig. 3
Forest plot of hospitalization rate comparing remdesivir to the control group in coronavirus disease 2019 patients. The forest plot indicates no significant difference in hospitalization rate between the remdesivir and control groups. CI, confidence interval.
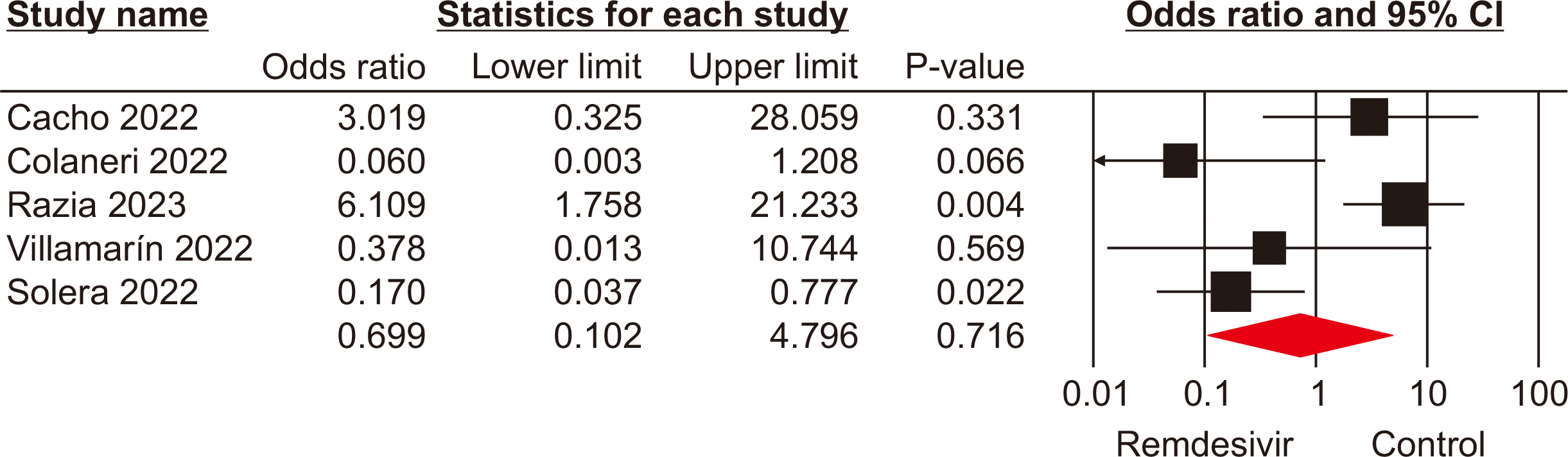
Fig. 4
Forest plot of intensive care unit admission comparing remdesivir to the control group in coronavirus disease 2019 patients. The forest plot indicates a significant difference in intensive care unit admission between the remdesivir and control groups. CI, confidence interval.
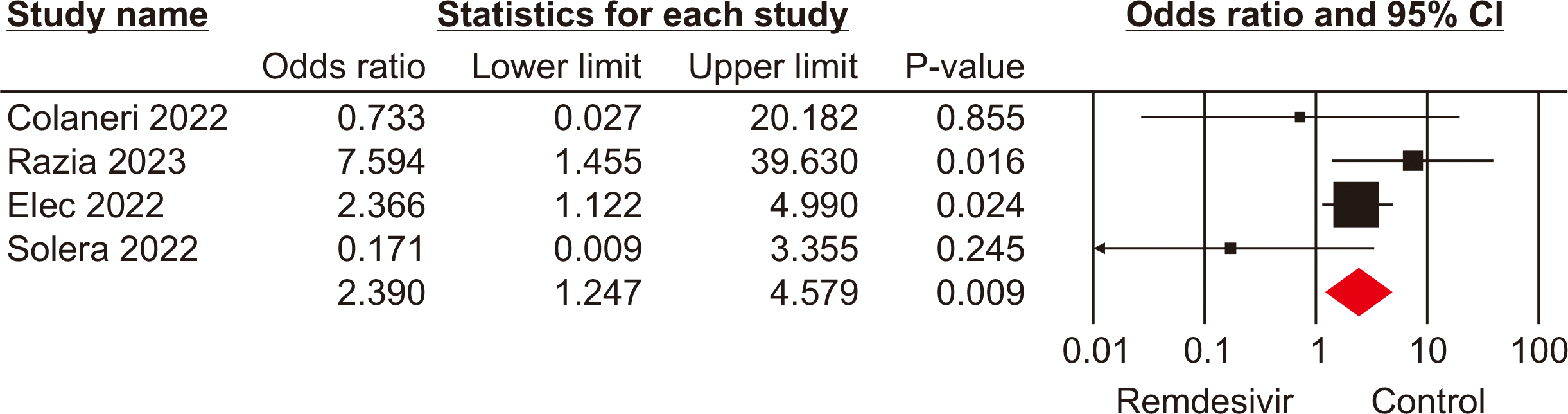
Fig. 5
Forest plot of need for mechanical ventilation comparing remdesivir to the control group in coronavirus disease 2019 patients. The forest plot indicates no significant difference in mechanical ventilation between the remdesivir and control groups. CI, confidence interval.
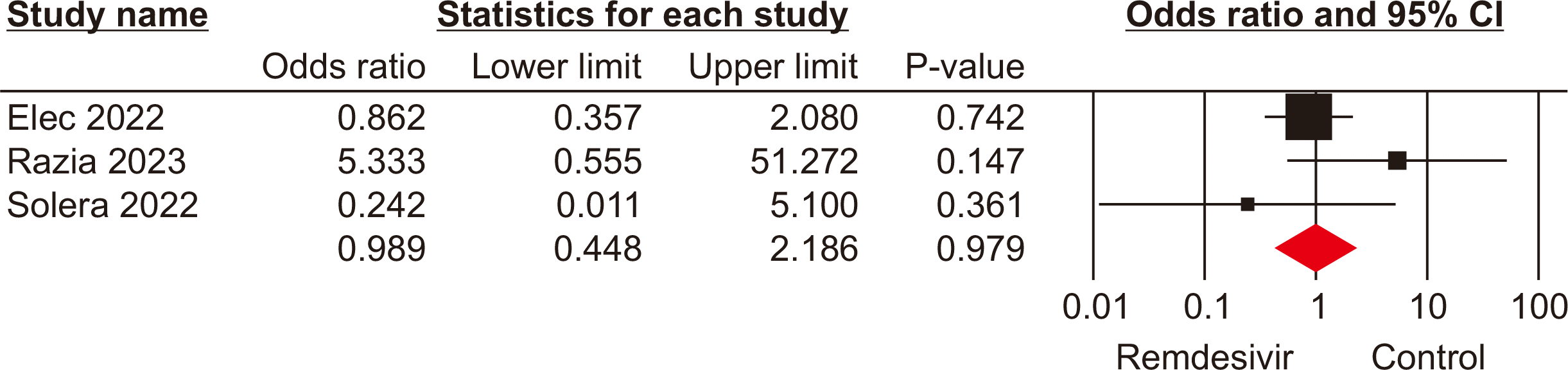
Table 1
Characteristics of studies included in the systematic review and meta-analysis
| Study | Country | Design | Data collection period | Transplant type | Sample size | Severity of COVID-19 | Remdesivir | No remdesivir | COVID-19 vaccination rate (%)a) | Reported outcomes | Treatment protocol |
Follow-up (day) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Mean age (yr) | No. of patients | Mean age (yr) | |||||||||||
| Cacho et al. (2022) [16] | Spain | RS | Nov 2021–Feb 2022 | Kidney | 98 | MS | 57 | 59 | 41 | 55 | 98 | Death, hospitalization, oxygen therapy | 2 or 4 | 30 |
| Colaneri et al. (2022) [23] | Italy | RS | Dec 2021–Feb 2022 | Kidney, heart, lung, liver | 24 | Moderate | 7 | 60 | 17 | 55 | 100 | Death, hospitalization, ICU admission | 3 | 28 |
| Elec et al. (2022) [24] | Romania | RS | Mar 2020–May 2021 | Kidney | 165 | MC | 35 | 53 | 127 | 49 | NA | Death, ICU admission, MV, oxygen therapy | 5 | NA |
| Fesu et al. (2022) [17] | Hungary | RS | Sep 2020–May 2021 | NA | 25 | NA | 15 | 55 | 10 | 49 | NA | Oxygen therapy | NA | 60 |
| Razia et al. (2023) [25] | USA | RS | Mar 2020–Aug 2022 | Lung | 54 | MM | 25 | 64 | 29 | 68 | NA | Death, hospitalization, ICU admission, MV | NA | 60 |
| Solera et al. (2023) [18] | Canada | RS | Apr–May 2022 | Kidney, heart, lung, liver, other | 192 | MM | 86 | 52 | 106 | 54 | 90 | Death, ICU admission, MV, oxygen therapy | 3 | 30 |
| Villamarín et al. (2022) [19] | Spain | RS | Jan–Apr 2022 | Kidney | 16 | Mild | 7 | 39 | 9 | 49 | 86 | Death, hospitalization, ICU admission | 3 | NA |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download