This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This retrospective observational matched cohort study assessed the differences in critical infections caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the omicron-predominant period of the coronavirus disease 2019 (COVID-19) pandemic. We evaluated the vaccine effectiveness of bivalent mRNA vaccine compared to unvaccinated individuals.
Methods
We collected COVID-19 case data from the Korean COVID-19 vaccine effectiveness cohort. We calculated the probability of critical COVID-19 cases by comparing the vaccinated and unvaccinated groups.
Results
The risk of being critically infected due to SAR-CoV-2 infection was 5.96 times higher (95% confidence interval, 5.63–6.38) among older individuals who were unvaccinated compared to those who received the bivalent COVID-19 vaccine.
Conclusion
Our findings indicate that the bivalent vaccine reduces the disease burden of the SARS-CoV-2 omicron variant, particularly among the older population. Further studies are warranted to determine the effectiveness of booster doses of vaccines for SARS-CoV-2 infection.
Keywords: SARS-CoV-2, Vaccine, Bivalent, COVID-19, Older People, Vaccine Effectiveness
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible virus with alpha, beta, gamma, delta, and omicron variants. Vaccines for coronavirus disease 2019 (COVID-19) are the best available interventions to reduce the public health impact of SARS-CoV-2 epidemics and pandemics.
1 The adenoviral vectored monovalent messenger ribonucleic acid (mRNA) vaccines have been used against the original SARS-CoV-2 strains for adults in the Republic of Korea since February 2021. In January 2022, the SARS-CoV-2 omicron variant overtook the delta variant to become the predominant COVID-19 strain in Korea,
23 and many SARS-CoV-2 infections developed during the omicron-predominant period.
3 Since October 2022, the bivalent (original and omicron BA.4/BA.5) vaccine replaced the monovalent vaccine in Korea.
Previous studies have demonstrated that bivalent COVID-19 vaccines are associated with an increased immune response and offer broader protection against the omicron variant compared to the monovalent mRNA COVID-19 vaccine.
45 Furthermore, a recent study demonstrated that a booster dose of the bivalent vaccine is more effective against SARS-CoV-2 infection and COVID-19-related hospital admission than a booster dose of the monovalent vaccine, regardless of previous SARS-CoV-2 infection history.
6 However, benefits of the bivalent vaccine against an omicron breakthrough infection—compared to no vaccine or an incomplete monovalent vaccine—remain unclear for a nationwide population.
This study therefore evaluated the difference in critical SARS-CoV-2 infection and death between individuals who received the bivalent mRNA COVID-19 vaccine and the unvaccinated group in a demographically matched population during the period of omicron predominance.
METHODS
This retrospective observational matched-cohort study compared individuals who received and did not receive the bivalent mRNA vaccine to evaluate the effectiveness of COVID-19 vaccination. We collected data on all COVID-19 cases from the Korean COVID-19 Vaccine Effectiveness (K-COVE) cohort. This cohort includes all critically infected COVID-19 cases extracted from the COVID-19 national surveillance system, which involves mandatory reporting, as well as patients’ COVID-19 vaccination records extracted from the National Immunization Registry.
As per the COVID-19 Response Guidelines in Korea, we defined COVID-19-confirmed cases as individuals who tested positive for SARS-CoV-2 based on the polymerase chain reaction test, rapid antigen test performed by medical experts, and emergency screening (emergency use-approved products).
7 We defined critical infection as severe individuals treated with high-flow oxygen therapy, mechanical ventilation, extracorporeal membrane oxygenation, or continuous renal replacement therapy and those who died within 28 days of a confirmed SARS-CoV-2 infection. Detailed information regarding the K-COVE cohort is described elsewhere.
89
From the K-COVE cohort, we included individuals aged ≥18 years who were infected at least once during the omicron-predominant period in this study. We defined unvaccinated individuals as those who did not receive any dose of the COVID-19 vaccine or received only a single dose of the monovalent COVID-19 vaccine (i.e., incomplete COVID-19 vaccination). We also defined vaccinated individuals as those who had received a vaccine within the previous 14 days and bivalent recipients as those who had received their bivalent vaccine at least 14 days earlier. We excluded individuals who had received more than two bivalent vaccine doses. Using the propensity score matching method, recipients who had received the bivalent vaccine were matched at a 1:1 ratio with unvaccinated individuals based on demographic factors, including sex, age, place of residence, previous infection history, residence in an older care facility, and immunocompromised status.
We calculated the proportion of critically infected COVID-19 patients and estimated the 95% confidence intervals (CIs) using the exact binomial method.
10 We assessed vaccine effectiveness (i.e., reduction in critical SARS-CoV-2 infection during the omicron-predominant period) for both the vaccinated and unvaccinated groups.
In theory, vaccine effectiveness is also obtained using the following formula
11:
where Relative Risk is the relative risk of developing a critical infection for the vaccinated vs. the unvaccinated group. All analyses were conducted using R version 4.2.1.
Ethics statement
This study was conducted under the Korea Infectious Diseases Control and Prevention Act (No. 12444 and No. 13392) with approval from the Institutional Review Board (IRB) of Korea Disease Control and Prevention Agency (IRB No. 2021-12-03-C-A). The requirement for informed consent was waived due to the retrospective study design.
RESULTS
We found that 30,133,884 individuals were infected with SARS-CoV-2 in the K-COVE cohort between January 16, 2022, and August 2, 2023. Among them, 1,560,742 individuals met the eligibility criteria and were included in the analysis. We identified a matched sample size of 291,707 individuals in the vaccinated and unvaccinated groups who experienced SARS-CoV-2 infection during the omicron-dominant period (
Supplementary Fig. 1).
Unvaccinated individuals were more likely to be critically infected compared with vaccinated individuals (
Fig. 1A,
Supplementary Tables 1 and
2). Unvaccinated older individuals were 5.96 times more likely to be critically infected (1.73%; 95% CI, 1.66–1.80%) than older individuals who had received the vaccine (0.29%; 95% CI, 0.26–0.32%).
Fig. 1
Probability of critical infection and bivalent COVID-19 vaccine effectiveness against severe SARS-CoV-2 infection and death in the Republic of Korea. (A) Proportion of individuals critical infection due to SARS-CoV-2 during the omicron wave of the COVID-19 pandemic by demographic characteristic, including sex, age, and history of SARS-CoV-2 infection. (B) Vaccine effectiveness against critical infection due to SARS-CoV-2 compared to those who did not receive the COVID-19 vaccine. The points are mean estimates, and the vertical bars indicate 95% confidence intervals.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, COVID-19 = coronavirus disease 2019.
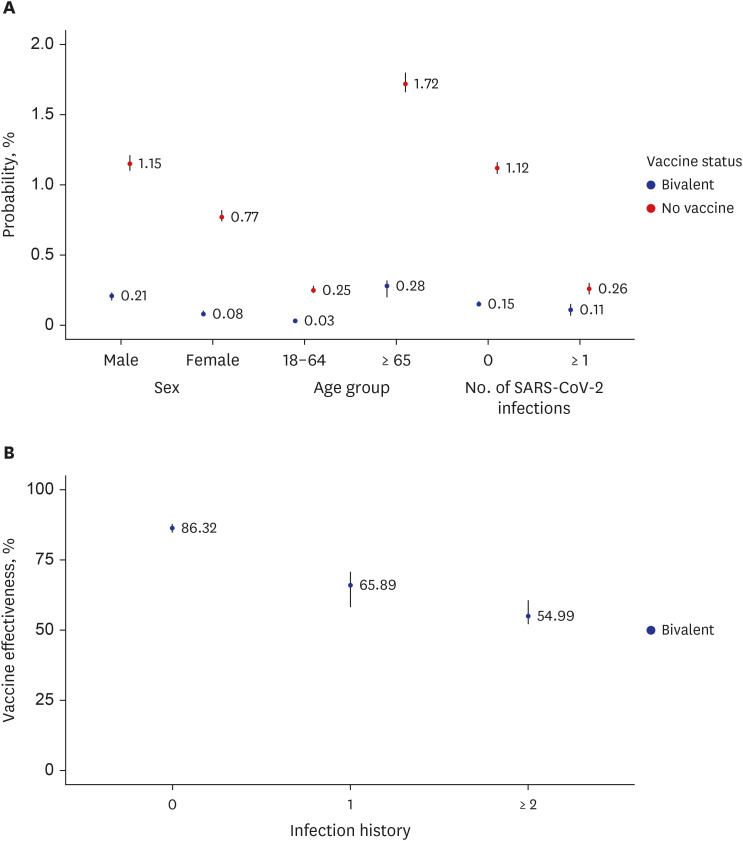
The bivalent vaccine’s effectiveness against critical infection was 86.32% (95% CI, 85.72–87.82%) and 65.89% (95% CI, 58.31–70.72%) for individuals with no history of SARS-CoV-2 infection and a history of one infection, respectively, compared with the unvaccinated group. The vaccine effectiveness was 54.99% (95% CI, 52.15–60.71%) for patients with a history of two or more SARS-CoV-2 infections (
Fig. 1B,
Supplementary Table 3).
DISCUSSION
This nationwide, cohort-matched study explored the effectiveness of the bivalent mRNA COVID-19 vaccine against severe SARS-CoV-2 infection and death. Those who had acquired immunity from the bivalent COVID-19 vaccine had lower risk of critical infection, particularly in the older population aged 65 years and above. This is in line with previous studies finding that the bivalent COVID-19 vaccine is more effective against hospitalization and death from SARS-CoV-2 infection among older people compared to young adults.
12 This is expected because older individuals have a weaker humoral immune response against SAR-CoV-2 infection compared to young adults. Therefore, COVID-19 vaccination improves the immune response more in older individuals.
13
We found that individuals with a history of SARS-CoV-2 infection had lower vaccine effectiveness than those with no history of SARS-CoV-2 infection. This is likely because the comparison group (i.e., individuals who previously had SARS-CoV-2 infection) had better immunogenicity than individuals with no history of SARS-CoV-2 infection.
14 Moreover, we found no significant differences in the number of previous SARS-CoV-2 infections in terms of vaccine effectiveness. However, the mean of vaccine effectiveness was lower in patients who had previously had two or more SARS-CoV-2 infections than in those with a history of one infection. This is likely due to the small sample size of individuals who previously had two or more infections included in the study. Hybrid immunity can provide a stronger immune response against SARS-CoV-2 infection than immunity acquired from SARS-CoV-2 infection or COVID-19 vaccination alone.
14 Therefore, further studies are warranted to identify the vaccine’s effectiveness in terms of hybrid immunity acquired from both natural infection and vaccination, as we are currently in the transition period of the acute COVID-19 pandemic.
This study has several limitations. First, we could not control for specific comorbidities due to lack of accessible data. Second, we did not consider asymptomatic SARS-CoV-2 infections, which may not have been reported in the public health surveillance system.
15 Third, we could not control for waning vaccine effectiveness due to limited data.
We found that the risk of being critically infected with the omicron variant of SARS-CoV-2 was lower in those who had received the bivalent COVID-19 vaccine, particularly among older people. This suggests that a bivalent booster COVID-19 vaccine reduces the disease burden of the omicron variant infection in the older population. Further studies controlling for demographic factors, including underlying diseases and the time between vaccination and SARS-CoV-2 infection, may clarify the benefits of bivalent vaccination.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download