Abstract
Objectives.
In this study, we evaluated the associations between birth-related exposures, postnatal factors, and the risk of allergic rhinitis and asthma in children and adolescents.
Methods.
We performed a comprehensive search of five literature databases up to May 2023. To quantify the associations of birth-related exposures (birth weight, delivery mode, prematurity, sex, maternal age, and parental allergy history) and postnatal factors (birth order, number of siblings, breastfeeding exclusivity, and breastfeeding duration) with allergic disease, we calculated pooled odds ratios and 95% confidence intervals. We conducted subgroup analyses for allergic disease type, birth order, number of siblings, and parental allergy history. The methodological quality of the identified studies was evaluated using the Newcastle-Ottawa Scale.
Results.
This meta-analysis included 31 studies, encompassing 218,899 patients in total. The birth-related exposures of low birth weight, maternal age, and prematurity (less than 37 weeks gestation) were not significantly associated with the risk of asthma or allergic rhinitis during childhood or adolescence. Male sex, family history of allergy, and cesarean delivery were linked to an elevated risk of asthma or allergic rhinitis. Among postnatal factors, exclusive breastfeeding, breastfeeding for longer than 6 months, second or later birth order, and having siblings exhibited protective effects against allergic diseases in offspring.
Conclusion.
The risks of allergic rhinitis and asthma were elevated in male patients, those delivered by cesarean section, and those with a family history of allergy. Conversely, exclusive breastfeeding, breastfeeding for longer than 6 months, and having siblings corresponded to a reduced risk of respiratory allergic diseases.
A common link between asthma and rhinitis is a predisposition to developing hypersensitivity reactions to environmental allergens, especially those present in the air [1]. The rising prevalence of asthma and allergic rhinitis in recent decades is well-documented [2]. These increases cannot be attributed solely to genetic factors, as environmental changes also play a role in the emergence of these conditions [3]. Evidence suggests that exposure during the early stages of life has a profound impact, indicating that the developing immune system may be particularly vulnerable to improper “programming” during this period [4]. Furthermore, an atopic phenotype may be established in utero [5]. The immune system begins responding to common environmental allergens early in development, and sensitization to these allergens could potentially occur even during the fetal stages [6]. Consequently, the allergen-specific responses of the human immune system may exhibit certain biases from birth that intensify with age. Accordingly, understanding and managing both prenatal and postnatal risk factors for diseases such as asthma and allergic rhinitis in children and adolescents could greatly contribute to reducing the global health burden of airway diseases. Although numerous studies have explored this topic, to our knowledge, no meta-analysis of perinatal risk factors has yet been published. Moreover, several conflicting reports are available regarding the impact of various birth-related exposures and postnatal factors on asthma and rhinitis [4,7-36]. Since medical prevention can be more effective than treatment, it is crucial to gather objective and high-quality evidence by synthesizing and analyzing these studies through meta-analysis. It is also vital to ascertain the degree of association of each perinatal risk factor with asthma and rhinitis. In this meta-analysis, we examined the risks of allergic rhinitis and asthma associated with a variety of birth-related and postnatal factors.
The necessity for Institutional Review Board (IRB) approval is not mandated by our institution in the case of a systematic review and meta-analysis exclusively reliant on published literature. This systematic review and meta-analysis was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [37]. This study protocol was prospectively registered in the Open Science Framework (https://osf.io/k7fgy/).
Reports were retrieved by searching the PubMed, Scopus, Embase, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) databases until February 2023. The key search Medical Subject Headings (MeSH) terms were as follows: “allergic rhinitis,” “hay fever,” “allergic rhinoconjunctivitis,” “seasonal allergic rhinitis,” “asthma,” “allergic diseases,” “pregnant women,” “pregnant,” “cesarean section,” “c-section,” “delivery mode,” “abdominal deliveries,” “post cesarean section,” “adolescent,” “child,” “breast feeding,” and “risk factors.” Detailed keywords and search methods retrieved from the database are listed in Supplementary Table 1. Two authors (SHH and HS) independently reviewed and selected candidate studies based on reviewing the title, abstract, and main text. If the two reviewers differed in their decision whether to include a study, it was decided through discussion with a third reviewer.
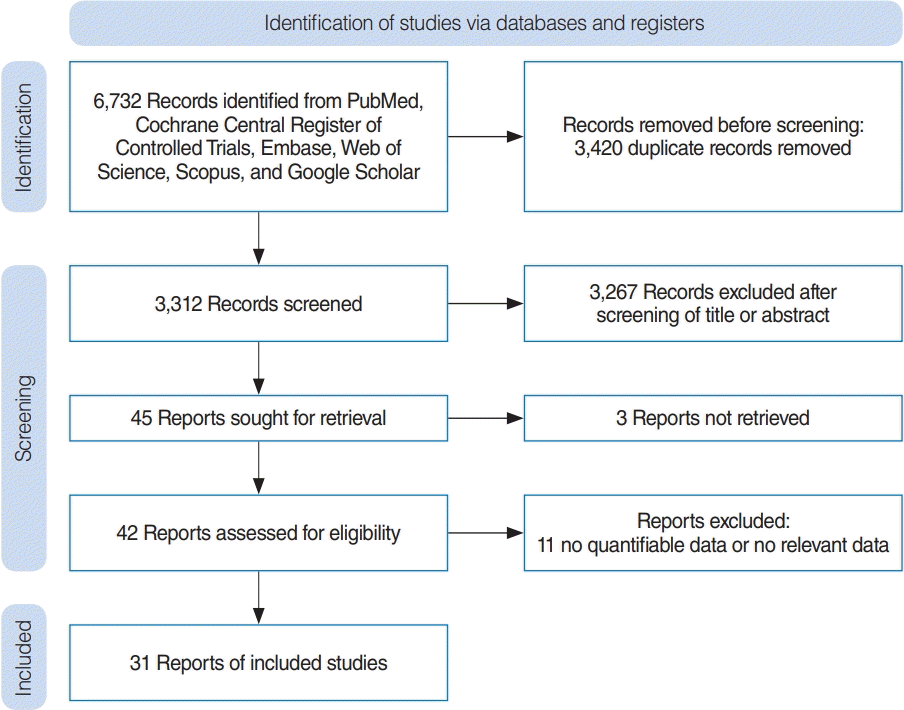
The analysis included cross-sectional, cohort, and case–control studies assessing the associations between birth-related exposures (birth weight, mode of delivery, prematurity, sex, maternal age, and parental history of allergy) and postnasal factors (birth order, number of siblings, and exclusiveness and duration of breastfeeding) and risks for allergic rhinitis and asthma in offspring (children and adolescents). In the literature, if allergic rhinitis or asthma was not specified, it was collectively referred to as “allergic diseases.” All study designs except case reports and review articles were included. These studies should have relevant data prediction, including risk ratio (odds ratio [OR]) and 95% confidence interval (CI). All studies were performed in human subjects. Articles with insufficient data for statistical analysis were excluded from the study. The selection strategy is summarized in Fig. 1.
Data were extracted from selected studies and items were organized in a standardized format [38]. We examined the following items: number of patients, sex, nationality, the presence of allergic rhinitis or asthma, and the OR and corresponding 95% CI to evaluate the relations of the risk factors with prevalence of allergic disease [4,7-36,39]. The risk of bias of the studies included in the analysis was assessed using the Newcastle-Ottawa scale.
R version 4.2.2 (R Foundation for Statistical Computing) was used for R version 4.2.2 (R Foundation for Statistical Computing) was used for statistical analysis. OR was used as the effect index. The correlation strengths of birth-related exposures and postnasal factors with respiratory allergic disease in offspring were determined by combining OR and 95% CI. The I2 test was used to assess heterogeneity. If both multivariate and univariate analyses were used to evaluate the risk factors, the OR and relevant 95% CI derived from multivariate analysis were used. The I2 test accounts for the rate of variability across studies due to heterogeneity with values ranging from 0 to 100 where higher values correspond to increased heterogeneity. In cases where a notable degree of heterogeneity was observed between the outcomes (I2 >50), a meta-analysis was conducted using a randomeffects model. On the other hand, for outcomes without significant heterogeneity (I2 <50), a fixed-effects model was used for the analysis. All P-values are reported as two-tailed values. Furthermore, sensitivity analyses were performed to assess the influence of individual studies on the overall findings of the metaanalysis. Potential publication bias for each item was identified using a combination of funnel plot and Egger’s test. When publication bias was suspected, the funnel plot asymmetry was corrected and confirmed by adding Duval and Tweedie’s trim-andfill method.
In total, 218,899 patients from 31 studies were included in the analysis. Study characteristics and an assessment of bias are provided in Table 1 [4,7-21,23-36,39] and Supplementary Table 2, respectively.
Low birth weight (OR, 0.9644; 95% CI, 0.8004–1.1622; I2=49.0%), maternal age above 35 years (OR, 0.8827; 95% CI, 0.7052–1.1049; I2=61.4%), and prematurity (defined as less than 37 weeks gestation; OR, 1.0189; 95% CI, 0.9156–1.1338; I2=14.9%) were not significantly associated with the risk of asthma or allergic rhinitis during childhood or adolescence. In contrast, male sex (OR, 1.4985; 95% CI, 1.3961–1.6084; I2=56.0%), family history of allergy (OR, 2.3300; 95% CI, 1.9690–2.7571; I2=92.2%), and cesarean delivery (OR, 1.2252; 95% CI, 1.1543–1.3004; I2=50.5%) did correspond to elevated risks of developing asthma or allergic rhinitis (Supplementary Fig. 1).
The Begg funnel plot analysis and the Egger test for prematurity (P=0.563), male sex (P=0.8109), and family history of allergy (P=0.0815) indicated no potential publication bias in these studies. However, the same tests for cesarean section (P=0.004629) suggested the presence of potential bias. Nevertheless, the trim-and-fill test revealed a lack of statistical significance when comparing observed and adjusted values (1.2252 [P<0.0001] vs. 1.1626 [P<0.0001]), leading us to conclude that no publication bias was present for the variables in the analysis. The results of the Begg funnel plot analysis are displayed in Supplementary Fig. 2. Due to the limited number of studies available (fewer than 10), we could not examine publication bias for low birth weight or maternal age above 35 years.
In a subgroup analysis comparing ORs by disease type (allergic rhinitis vs. asthma), the associations of birth-related exposures were found to be similar between conditions. Specifically, birth weight (allergic rhinitis, 0.8065 [95% CI, 0.6353–1.0237] vs. asthma, 1.2355 [95% CI, 0.9069–1.6831]; P=0.4538), cesarean delivery (allergic rhinitis, 1.2216 [95% CI, 1.1159–1.3374] vs. asthma, 1.2270 [95% CI, 1.0991–1.3698]; P=0.9516), prematurity (allergic rhinitis, 1.0109 [95% CI, 0.9056–1.1283] vs. asthma, 1.1418 [95% CI, 0.7096–1.8372]; P=0.7941), sex (allergic rhinitis, 1.5331 [95% CI, 1.4317–1.6417] vs. asthma, 1.5590 [95% CI, 1.4244–1.7064]; P=0.3259), maternal age (allergic rhinitis, 0.9273 [95% CI, 0.7139–1.2044] vs. asthma, 0.7289 [95% CI, 0.3877–1.3707]; P=0.4901), and parental history of allergy (allergic rhinitis, 2.5807 [95% CI, 2.0509–3.2474] vs. asthma, 1.9223 [95% CI, 1.2784–2.8904]; P=0.2995) showed no significant differences between allergic rhinitis and asthma in their causative or preventive associations (Supplementary Fig. 1).
In a subgroup analysis examining family history, the risk of allergic disease was found to be higher for a maternal history of allergy (OR, 2.5191; 95% CI, 2.0369–3.1155) compared to a paternal history (OR, 1.6880; 95% CI, 1.2735–2.2375; P=0.0262). However, we observed no significant differences in the risk of allergic disease when comparing a history of allergy in a single parent (OR, 2.1035; 95% CI, 1.8149–2.4380) to a biparental history (OR, 2.0901; 95% CI, 1.8113–2.4119; P=0.9516) (Supplementary Fig. 1).
Exclusive breastfeeding (OR, 0.7573; 95% CI, 0.6564–0.8738; I2=73.7%) and a breastfeeding duration exceeding 6 months (OR, 0.8584; 95% CI, 0.7907–0.9319; I2=67.3%) were associated with a reduced risk of allergic disease in offspring. Additionally, second or later birth order (OR, 0.7925; 95% CI, 0.7526– 0.8344; I2=66.3%), and the presence of siblings (OR, 0.7836; 95% CI, 0.7300–0.8412; I2=55.9%) were also negatively associated with the development of allergic disease (Supplementary Fig. 3).
The Begg funnel plot and Egger test for exclusive breastfeeding (P=0.07949), a breastfeeding duration exceeding 6 months (P=0.9796), and the presence of siblings (P=0.3667) indicated no evidence of publication bias in the studies. However, the Egger test (P=0.004675) and Begg funnel plot analysis for second or later birth order (P=0.03244) did suggest the possibility of publication bias.
In a subgroup analysis comparing ORs by disease type (allergic rhinitis vs. asthma), postnatal factors such as exclusive breastfeeding (allergic rhinitis, 0.7471 [95% CI, 0.6317–0.8836] vs. asthma, 0.7724 [95% CI, 0.5300–1.1255]; P=0.8746) and a breastfeeding duration exceeding 6 months (allergic rhinitis, 0.8306 [95% CI, 0.7600–0.9076] vs. asthma, 0.9374 [95% CI, 0.8047–1.0920]; P=0.1793) were associated with similar causative or preventive effects on both allergic rhinitis and asthma. However, second or later birth order (allergic rhinitis, 0.7369 [95% CI, 0.6796–0.7989] vs. asthma, 0.8278 [95% CI, 0.6863– 0.9986]; P=0.0048) and the presence of siblings (allergic rhinitis, 0.7477 [95% CI, 0.6910–0.8090] vs. asthma, 0.8618 [95% CI, 0.6735–1.1026]; P=0.0218) were found to exert greater preventive effects against allergic rhinitis than against asthma (Supplementary Fig. 3).
Regarding birth order and the number of siblings, we observed no significant differences in the risk of allergic disease between individuals born second (OR, 0.8185; 95% CI, 0.7670–0.8733) and those born third or later (OR, 0.7535; 95% CI, 0.6827–0.8316; P=0.1701). Relative to those without siblings, participants with one sibling displayed a reduced risk of allergic disease (OR, 0.8341; 95% CI, 0.7515–0.9257), which was comparable to that of participants with two or more siblings (OR, 0.7659; 95% CI, 0.7020–0.8357; P=0.2187) (Supplementary Fig. 3).
Through the meta-analysis employed in this study, we were able to conduct an OR analysis that statistically integrated data from a large patient cohort, thereby providing more objective and robust evidence compared to the individual studies. Additionally, we could determine the magnitude of the OR for each factor. Among birth-related exposures, family history of allergy exhibited the highest OR for developing asthma or allergic rhinitis, followed by male sex and cesarean delivery. Within the context of family history, a maternal history of allergy was particularly influential. Among postnatal factors, exclusive breastfeeding showed the strongest negative association, followed by the presence of siblings, second or later birth order, and a longer duration of breastfeeding (exceeding 6 months). Notably, the presence of siblings and birth order were more negatively associated with allergic rhinitis than asthma. Studies have suggested that allergic rhinitis and asthma are linked to low birth weight, greater maternal age, and prematurity [14,17,21]. However, our analysis revealed that while low birth weight displayed a negative association with allergic rhinitis, it was positively related to asthma. When these findings were synthesized, we concluded that no overall relationship was present. The evidence regarding advanced maternal age and prematurity was inconsistent, but our analysis determined that their relationship with allergic disease was not definitive.
In the subgroup analysis, second or later birth order and the presence of siblings demonstrated stronger preventive effects against allergic rhinitis than against asthma. However, all birth-related exposures—birth weight, cesarean delivery, prematurity, sex, maternal age, and parental history of allergy—as well as postnatal factors such as exclusive or prolonged breastfeeding (exceeding 6 months) were found to exert similar causative or preventive influences on allergic rhinitis and asthma.
Allergic rhinitis and asthma are believed to be influenced by both genetic and environmental factors [40]. These prevalent allergic diseases share similar etiologies and often co-occur within families [41]. The genetic predisposition to allergic diseases is well-documented, highlighting why a family history of these conditions is a key risk factor for the development of allergies in children [42]. Recently, however, the distinct nature of these two diseases has been more actively debated, shifting away from the “one airway, one disease” concept [43]. In the present study, we confirmed that sibling interactions were more closely associated with allergic rhinitis than with asthma. Children who are exposed to familial infections or gut commensals may encounter microorganisms more frequently and in larger quantities, thereby facilitating appropriate immune conditioning. Moreover, increased levels of household crowding have been linked to higher antigen exposure, which may contribute to the protective effect of crowding observed [4,35]. It seems that continuous exposure to allergens may play a greater role in the development of allergic rhinitis than in asthma, warranting further investigation into this matter.
In this study, we found that boys faced higher risks for allergic rhinitis and asthma during childhood. However, research suggests that the prevalence of these allergic diseases in girls and women increases with age [44]. This trend may be attributed to genetic susceptibility and environmental factors. Additionally, it has been proposed that hormonal influences, specifically estrogen and progesterone, could render women more susceptible to allergic diseases [44].
A previous multidisciplinary review yielded consistent findings on the relationship between breastfeeding and the development of allergic diseases [45]. The potential for breastfeeding to prevent allergies has been explored through various methods. These include analyzing traces of food proteins ingested by lactating mothers, which may promote tolerance to these foods, as well as the elimination of microbes that could trigger inflammatory responses [46].
Previous research has compared the effects of cesarean delivery with those of vaginal birth, considering factors such as the absence of vaginal compression on the neonate’s chest and the reduced stress associated with cesarean delivery. The stress and labor involved in vaginal delivery stimulate the release of catecholamines, cortisol, and pulmonary surfactant, which contribute substantially to normal postnatal lung development [47]. Furthermore, it has been postulated that cesarean section may hinder the maturation of the immune system by not exposing the neonate to maternal vaginal microflora, potentially affecting the balance between Th1 and Th2 lymphocytes early in life [48]. Cesarean section rates are on the rise globally, for both medical and nonmedical reasons [49]. However, cesarean section may also impact lactogenesis, decreasing the likelihood or delaying the onset of breastfeeding [50]. Therefore, the nonmedical decision to undergo cesarean section and the duration and extent of breastfeeding should be considered important elements of maternal education, as they could influence the development of asthma and allergic rhinitis in children.
This study had several limitations. First, most research exploring the links between prenatal or postnatal factors and respiratory allergies in children and adolescents has employed retrospective or cross-sectional designs. These studies often lack explicit clinical criteria for maternal or medical decision-making regarding cesarean section, preterm birth, maternal age, breastfeeding, and other relevant factors. As a result, our ability to draw cause-and-effect conclusions was constrained. To address this gap, further clinical studies are needed to assess the impact of prenatal and postnatal factors on the development of respiratory allergies. Second, variations in publication domains, study durations, and approaches used to address confounding variables may lead to discrepancies between the reported results and the actual underlying conditions. Additionally, our analysis included studies that were based on self-reported diagnoses of allergic rhinitis or asthma. This reliance on self-reporting could lead to differences from the actual prevalence rates, thereby limiting the generalizability of our findings. Crucially, any errors or inaccuracies within the individual studies may have impacted our broader analysis. Despite these limitations, the robust association observed between prenatal or postnatal factors and the development of respiratory allergies underscores the need for future randomized trials. Such trials should investigate the potential influence of these factors on respiratory allergic conditions.
In summary, allergic rhinitis and asthma displayed higher incidence rates among individuals who were delivered by cesarean section, those identified as male at birth, and those with a family history of allergies. Conversely, exclusive and prolonged breastfeeding (lasting more than 6 months) and the presence of siblings may represent protective factors against the development of allergic rhinitis and asthma in children and adolescents.
▪Male sex, a family history of allergy, and delivery by cesarean section were associated with an increased risk of developing asthma or allergic rhinitis.
▪ Exclusive breastfeeding and a prolonged duration of breastfeeding (beyond 6 months) demonstrated preventive effects against allergic diseases in offspring.
▪ Both second or later birth order and the presence of sibling(s) were associated with a reduced risk of developing respiratory allergic disease.
Notes
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00209494, 2022R1F1A1066232), as well as a Korean Fund for Regenerative Medicine (KFRM) grant also funded by the Korea government (23C0121L1).
Supplementary materials
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2024.00024.
Supplementary Fig. 1.
Odd ratios of birth-related exposures associated with risk of allergic rhinitis and asthma. (A) Low birth weight. (B) Maternal age above 35 years. (C) Prematurity (under 37 weeks of gestation).
(D) Male sex. (E) Family history of allergy.
(F) Cesarean delivery. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval.
Supplementary Fig. 2.
Begg funnel plot analyses for various factors. (A) Prematurity. (B) Male sex. (C) Family history of allergy. (D) Cesarean delivery. (E) Exclusive breastfeeding. (F) Prolonged breastfeeding duration (greater than 6 months). (G) Presence of one or more siblings. (H) Birth order.
Supplementary Fig. 3.
Odds ratios of postnatal factors associated with risk of allergic rhinitis and asthma. (A) Exclusive breastfeeding. (B) Prolonged breastfeeding duration (greater than 6 months).
(C) Second or later birth order. (D) Presence of one or more siblings. TE, estimate of treatment effect; seTE, standard error of treatment estimate; OR, odds ratio; CI, confidence interval.
REFERENCES
1. Bousquet J, Schunemann HJ, Togias A, Bachert C, Erhola M, Hellings PW, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020; Jan. 145(1):70–80.
2. Gutowska-Slesik J, Samolinski B, Krzych-Falta E. The increase in allergic conditions based on a review of literature. Postepy Dermatol Alergol. 2023; Feb. 40(1):1–7.
3. D’Amato G, Vitale C, Lanza M, Molino A, D’Amato M. Climate change, air pollution, and allergic respiratory diseases: an update. Curr Opin Allergy Clin Immunol. 2016; Oct. 16(5):434–40.

4. Montgomery SM, Wakefield AJ, Morris DL, Pounder RE, Murch SH. The initial care of newborn infants and subsequent hay fever. Allergy. 2000; Oct. 55(10):916–22.

5. Xu B, Jarvelin MR, Pekkanen J. Prenatal factors and occurrence of rhinitis and eczema among offspring. Allergy. 1999; Aug. 54(8):829–36.

6. Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D’Vaz N, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. 2011; Feb. 127(2):470–8.

7. Bager P, Melbye M, Rostgaard K, Benn CS, Westergaard T. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. 2003; Jan. 111(1):51–6.

8. Bedolla-Barajas M, Javier Ramirez-Cervantes F, Morales-Romero J, Jesus Perez-Molina J, Meza-Lopez C, Delgado-Figueroa N. A rural environment does not protect against asthma or other allergic diseases amongst Mexican children. Allergol Immunopathol (Madr). 2018; Jan-Feb. 46(1):31–8.

9. Bion V, Lockett GA, Soto-Ramirez N, Zhang H, Venter C, Karmaus W, et al. Evaluating the efficacy of breastfeeding guidelines on longterm outcomes for allergic disease. Allergy. 2016; May. 71(5):661–70.

10. Chu S, Zhang Y, Jiang Y, Sun W, Zhu Q, Wang B, et al. Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by breastfeeding. Sci Rep. 2017; Aug. 7(1):9762.

11. Ehlayel MS, Bener A. Duration of breast-feeding and the risk of childhood allergic diseases in a developing country. Allergy Asthma Proc. 2008; Jul-Aug. 29(4):386–91.

12. Gorris A, Bustamante G, Mayer KA, Kinaciyan T, Zlabinger GJ. Cesarean section and risk of allergies in Ecuadorian children: a crosssectional study. Immun Inflamm Dis. 2020; Dec. 8(4):763–73.

13. Han DH, Shin JM, An S, Kim JS, Kim DY, Moon S, et al. Long-term breastfeeding in the prevention of allergic rhinitis: Allergic Rhinitis Cohort Study for Kids (ARCO-Kids study). Clin Exp Otorhinolaryngol. 2019; Aug. 12(3):301–7.

14. Hu Y, Chen Y, Liu S, Jiang F, Wu M, Yan C, et al. Breastfeeding duration modified the effects of neonatal and familial risk factors on childhood asthma and allergy: a population-based study. Respir Res. 2021; Feb. 22(1):41.

15. Goycochea Valdivia WA, Hidalgo Tunque CM, Hernandez Diaz H, Centeno Huaman J. Association among prematurity, low birth weight and exclusive breastfeeding with allergic rhinitis in 2 to 7 year-old pediatric patients from Hospital Nacional Cayetano Heredia, Peru. Bol Med Hosp Infant Mex. 2010; Aug. 67(4):315–26.
16. Jelding-Dannemand E, Malby Schoos AM, Bisgaard H. Breast-feeding does not protect against allergic sensitization in early childhood and allergy-associated disease at age 7 years. J Allergy Clin Immunol. 2015; Nov. 136(5):1302–8.

17. Kim HI, Nam S, Park Y, Jung YJ, Kim HY, Kim KW, et al. Cesarean section does not increase the prevalence of allergic disease within 3 years of age in the offsprings. Obstet Gynecol Sci. 2019; Jan. 62(1):11–8.

18. Krzych-Falta E, Furmanczyk K, Lisiecka-Bielanowicz M, Sybilski A, Tomaszewska A, Raciborski F, et al. The effect of selected risk factors, including the mode of delivery, on the development of allergic rhinitis and bronchial asthma. Postepy Dermatol Alergol. 2018; Jun. 35(3):267–73.

19. Li Y, Jiang Y, Li S, Shen X, Liu J, Jiang F. Pre- and postnatal risk factors in relation to allergic rhinitis in school-aged children in China. PLoS One. 2015; Feb. 10(2):e0114022.

20. Lu HY, Chiu CW, Kao PH, Tsai ZT, Gau CC, Lee WF, et al. Association between maternal age at delivery and allergic rhinitis in schoolchildren: a population-based study. World Allergy Organ J. 2020; Jun. 13(6):100127.

21. Mallen CD, Mottram S, Wynne-Jones G, Thomas E. Birth-related exposures and asthma and allergy in adulthood: a population-based cross-sectional study of young adults in North Staffordshire. J Asthma. 2008; May. 45(4):309–12.

22. Matheson MC, Erbas B, Balasuriya A, Jenkins MA, Wharton CL, Tang ML, et al. Breast-feeding and atopic disease: a cohort study from childhood to middle age. J Allergy Clin Immunol. 2007; Nov. 120(5):1051–7.

23. McKeever TM, Lewis SA, Smith C, Hubbard R. Mode of delivery and risk of developing allergic disease. J Allergy Clin Immunol. 2002; May. 109(5):800–2.

24. Meza-Lopez C, Bedolla-Barajas M, Morales-Romero J, JimenezCarrillo CE, Bedolla-Pulido TR, Santos-Valencia EA. Prevalence of allergic diseases and their symptoms in schoolchildren according to the birth mode. Bol Med Hosp Infant Mex. 2021; Mar. 78(2):130–5.
25. Miyake Y, Arakawa M, Tanaka K, Sasaki S, Ohya Y. Cross-sectional study of allergic disorders associated with breastfeeding in Japan: the Ryukyus Child Health Study. Pediatr Allergy Immunol. 2007; Aug. 18(5):433–40.

26. Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000; Nov. 106(5):867–73.

27. Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004; Feb. 15(1):48–54.

28. Park YH, Kim KW, Choi BS, Jee HM, Sohn MH, Kim KE. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol Res. 2010; Jan. 2(1):28–33.

29. Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by caesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008; Aug. 122(2):274–9.
30. Renz-Polster H, David MR, Buist AS, Vollmer WM, O’Connor EA, Frazier EA, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005; Nov. 35(11):1466–72.

31. Salam MT, Margolis HG, McConnell R, McGregor JA, Avol EL, Gilliland FD. Mode of delivery is associated with asthma and allergy occurrences in children. Ann Epidemiol. 2006; May. 16(5):341–6.

32. Tong H, Gao L, Deng Y, Kong Y, Xiang R, Tan L, et al. Prevalence of allergic rhinitis and associated risk factors in 6 to 12 years schoolchildren from Wuhan in Central China: a cross-sectional study. Am J Rhinol Allergy. 2020; Sep. 34(5):632–41.

33. Tong X, Tong H, Gao L, Deng Y, Xiang R, Cen R, et al. A multicenter study of prevalence and risk factors for allergic rhinitis in primary school children in 5 cities of Hubei province, China. Int Arch Allergy Immunol. 2022; 183(1):34–44.

34. Wang T, Shi H, Qi H, Jiang L, Lin Y, Yao J, et al. Parental, gestational, and early-life exposure to indoor environmental hazardous factors on allergic rhinitis among preschool children in Urumqi City: a case-control study. J Pediatr (Rio J). 2023; Jul-Aug. 99(4):348–54.

35. Westergaard T, Rostgaard K, Wohlfahrt J, Andersen PK, Aaby P, Melbye M. Sibship characteristics and risk of allergic rhinitis and asthma. Am J Epidemiol. 2005; Jul. 162(2):125–32.

36. Xu B, Pekkanen J, Hartikainen AL, Jarvelin MR. Caesarean section and risk of asthma and allergy in adulthood. J Allergy Clin Immunol. 2001; Apr. 107(4):732–3.

37. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; Jan. 4(1):1.

38. Hwang SH, Kim SW, Basurrah MA, Kim DH. Efficacy of steroid-impregnated spacers after endoscopic sinus surgery in chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2023; May. 16(2):148–58.

39. Brandao HV, Vieira GO, de Oliveira Vieira T, Camargos PA, de Souza Teles CA, Guimaraes AC, et al. Increased risk of allergic rhinitis among children delivered by cesarean section: a cross-sectional study nested in a birth cohort. BMC Pediatr. 2016; Apr. 16:57.

40. Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2016 revision. J Allergy Clin Immunol. 2017; Oct. 140(4):950–8.
41. Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002; Jan. 165(2):176–80.
42. Stikker BS, Hendriks RW, Stadhouders R. Decoding the genetic and epigenetic basis of asthma. Allergy. 2023; Apr. 78(4):940–56.

43. Bousquet J, Melen E, Haahtela T, Koppelman GH, Togias A, Valenta R, et al. Rhinitis associated with asthma is distinct from rhinitis alone: the ARIA-MeDALL hypothesis. Allergy. 2023; May. 78(5):1169–203.
44. Frohlich M, Pinart M, Keller T, Reich A, Cabieses B, Hohmann C, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood?: a meta-analysis. Clin Transl Allergy. 2017; Dec. 7:44.

45. Kneepkens CM, Brand PL. Clinical practice: breastfeeding and the prevention of allergy. Eur J Pediatr. 2010; Aug. 169(8):911–7.
46. Spiekermann GM, Walker WA. Oral tolerance and its role in clinical disease. J Pediatr Gastroenterol Nutr. 2001; Mar. 32(3):237–55.

47. Madar J, Richmond S, Hey E. Surfactant-deficient respiratory distress after elective delivery at ‘term’. Acta Paediatr. 1999; Nov. 88(11):1244–8.

48. Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy. 2018; Dec. 73(12):2314–27.

Table 1.
Characteristics of included studies
| First author (year) | Study design | Sample size | Age (yr) | Timeline (yr) | Sex (male:female) | Nation | Allergic disease diagnosis | Comparison |
|---|---|---|---|---|---|---|---|---|
| Nafstad (2000) [26] | Cohort | 2,531 | 4 | 4 | 1,298:1,233 | Sweden | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery), any family history of allergy, single-parent allergy history (mother vs. father), parental allergy history (single vs. both), sex (male vs. female), maternal age at birth (older than 35 vs. less than 35 years), birth order (second vs. third or later), number of siblings (2 vs. 3 or more) |
| Montgomery (2000) [4] | Cohort | 5,519 | 26 | 27 | NA | UK | Survey-based | Any family history of allergy, parental allergy history (single vs. both), birth order (second vs. third or later), number of siblings (2 vs. 3 or more), exclusive breastfeeding, breastfeeding duration exceeding 6 months, delivery mode (C-section vs. vaginal delivery) |
| Xu (2001) [36] | Cohort | 11,635 | 31 | 31 | NA | Finland | Physician-based | Delivery mode (C-section vs. vaginal delivery) |
| McKeever (2002) [23] | Cohort | 29,238 | 2.9 (0–11) | 2 | NA | UK | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery) |
| Bager (2003) [7] | Cohort | 9,722 | 25 (20–28) | 25 | 0:9,722 | Denmark | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery), maternal age at birth greater than 35 years, prematurity (<37 weeks), number of siblings (2 vs. 3 or more) |
| Negele (2004) [27] | Cohort | 2,500 | 2 | 2 | 1,205:1,295 | Germany | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery) |
| Renz-Polster (2005) [30] | Cohort | 7,872 | 3–10 | 3–10 | 4,001:3,871 | USA | Physician-based | Sex (male vs. female), allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery), birth order (second vs. third or later), number of siblings (2 vs. 3 or more) |
| Salam (2006) [31] | Cohort | 2,653 | 10.5±2.2 | 10.5 | NA | USA | Survey-based | Delivery mode (C-section vs. vaginal delivery), allergic disease (AR vs. asthma) |
| Miyake (2007) [25] | Cross-sectional | 24,077 | 6–15 | 6–15 | 12,161:11,916 | Japan | Survey-based | Allergic disease (AR vs. asthma), exclusive breastfeeding, breastfeeding duration exceeding 6 months |
| Westergaard (2005) [35] | Cross-sectional | 31,145 | 15–43 | 15–43 | All women | Denmark | Physician-based | Birth order (second vs. third or later), number of siblings (2 vs. 3 or more) |
| Ehlayel (2008) [11] | Cross-sectional | 1,278 | 0–5 | 0–5 | 632:646 | UK | Physician-based | Allergic disease (AR vs. asthma), exclusive breastfeeding |
| Mallen (2008) [21] | Cross-sectional | 567 | 18–25 | 18–25 | NA | UK | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery), prematurity (<37 weeks) |
| Pistiner (2008) [29] | Cohort | 432 | 7.4 (6.5–10.1) | 9 | 237:195 | USA | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery) |
| Park (2010) [28] | Cross-sectional | 279 | 4.6±3.8 | 4.6±3.8 | 180:99 | Korea | Physician-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery) |
| Goycochea Valdivia (2010) [15] | Case-control | 366 | 4.02 (2–7) | 2–7 | 172:197 | Peru | Physician-based | Exclusive breastfeeding, low birth weight (<2,500 g), prematurity (<37 weeks) |
| Jelding-Dannemand (2015) [16] | Cohort | 335 | 7 | 7 | NA | Denmark | Physician-based | Allergic disease (AR vs. asthma), exclusive breastfeeding |
| Li (2015) [19] | Cross-sectional | 20,803 | 9.19 | 9.19 | 10,803:10,000 | China | Survey-based | Exclusive breastfeeding, delivery mode (C-section vs. vaginal delivery), sex (male vs. female), prematurity (<37 weeks) |
| Bion (2016) [9] | Cohort | 2,140 | 10–18 | 10 | NA | UK | Survey-based | Allergic disease (AR vs. asthma), exclusive breastfeeding, breastfeeding duration exceeding 6 months |
| Brandao (2016) [39] | Cross-sectional | 672 | 6 | 6 | 336:336 | Brazil | Survey-based | Allergic disease (AR vs. asthma), delivery mode (C-section vs. vaginal delivery) |
| Chu (2017) [10] | Cross-sectional | 12,639 | 7–10 | 7–10 | NA | China | Survey-based | Delivery mode (C-section vs. vaginal delivery) |
| Bedolla-Barajas (2018) [8] | Cross-sectional | 1,003 | 6–7 | 6–7 | 474:529 | Mexico | Survey-based | Allergic disease (AR vs. asthma), any family history of allergy, parental allergy history (single vs. both), sex (male vs. female) |
| Krzych-Falta (2018) [18] | Cross-sectional | 18,617 | NA | 6–44 | 8,606:10,011 | Poland | Physician-based | Delivery mode (C-section vs. vaginal delivery), any family history of allergy |
| Han (2019) [13] | Cohort | 1,374 | 4–12 | 4–12 | 941:433 | Korea | Physician-based | Breastfeeding duration exceeding 6 months, delivery mode (C-section vs. vaginal delivery), number of siblings (2 vs. 3 or more) |
| Kim (2019) [17] | Cohort | 175 | 3 | 3 | 78:97 | Korea | Survey-based | Delivery mode (C-section vs. vaginal delivery), any family history of allergy, parental allergy history (single vs. both), low birth weight (<2,500 g), prematurity (<37 weeks), sex (male vs. female) |
| Gorris (2020) [12] | Cross-sectional | 189 | 8.2±2.7 | 8.2±2.7 | 101:88 | Austria | Physician-based | Allergic disease (AR vs. asthma), exclusive breastfeeding, delivery mode (C-section vs. vaginal delivery), any family history of allergy, single-parent allergy history (mother vs. father), parental allergy history (single vs. both), prematurity (<37 weeks), sex (male vs. female) |
| Lu (2020) [20] | Cohort | 1,344 | 6.4 | 6.4 | 763:581 | Taiwan | Physician-based | Delivery mode (C-section vs. vaginal delivery), any family history of allergy, single-parent allergy history (mother vs. father), parental allergy history (single vs. both), maternal age at birth greater than 35 years, prematurity (<37 weeks), birth order (second vs. third or later), number of siblings (2 vs. 3 or more), sex (male vs. female) |
| Tong (2020) [32] | Cross-sectional | 5,550 | 6–12 | 8.79±1.7 | 2,993:2,557 | China | Physician-based | Breastfeeding duration exceeding 6 months, any family history of allergy, maternal age at birth greater than 35 years, sex (male vs. female) |
| Hu (2021) [14] | Cohort | 10,464 | 6–12 | 9.2±2.2 | 5,464:5,000 | China | Survey-based | Allergic disease (AR vs. asthma), breastfeeding duration exceeding 6 months, delivery mode (C-section vs. vaginal delivery), any family history of allergy, low birth weight (<2,500 g), prematurity (<37 weeks), sex (male vs. female) |
| Meza-Lopez (2021) [24] | Cross-sectional | 1,003 | 6–7 | 6–7 | 477:526 | Mexico | Survey-based | Delivery mode (C-section vs. vaginal delivery) |
| Tong (2022) [33] | Cross-sectional | 10,757 | 6–12 | 8.91±1.78 | 5,809:4,948 | China | Survey-based | Breastfeeding duration exceeding 6 months, delivery mode (C-section vs. vaginal delivery), any family history of allergy, maternal age at birth greater than 35 years, sex (male vs. female) |
| Wang (2023) [34] | Case-control | 2,020 | 5.18±0.99 | 5.18±0.99 | 225:179 | China | Survey-based | Breastfeeding duration exceeding 6 months, delivery mode (C-section vs. vaginal delivery), any family history of allergy, single-parent allergy history (mother vs. father), parental allergy history (single vs. both), low birth weight (<2,500 g), prematurity (<37 weeks) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download