1. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: adult basic and advanced life support. 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020; 142(16_suppl_2):S366–468.
2. Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury: mechanisms and practical aspects. Nat Rev Neurol. 2012; 8:214–22.

3. Jackson TC, Kochanek PM. A new vision for therapeutic hypothermia in the era of targeted temperature management: a speculative synthesis. Ther Hypothermia Temp Manag. 2019; 9:13–47.

4. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009; 37:S186–202.

5. Schreckinger M, Marion DW. Contemporary management of traumatic intracranial hypertension: is there a role for therapeutic hypothermia? Neurocrit Care. 2009; 11:427–36.

6. Chen H, Wu F, Yang P, Shao J, Chen Q, Zheng R. A meta-analysis of the effects of therapeutic hypothermia in adult patients with traumatic brain injury. Crit Care. 2019; 23:396.

7. Yao Z, You C, He M. Effect and feasibility of therapeutic hypothermia in patients with hemorrhagic stroke: a systematic review and meta-analysis. World Neurosurg. 2018; 111:404–12.
8. Baker TS, Durbin J, Troiani Z, Ascanio-Cortez L, Baron R, Costa A, et al. Therapeutic hypothermia for intracerebral hemorrhage: systematic review and meta-analysis of the experimental and clinical literature. Int J Stroke. 2022; 17:506–16.

9. Kuczynski AM, Marzoughi S, Al Sultan AS, Colbourne F, Menon BK, van Es AC, et al. Therapeutic hypothermia in acute ischemic stroke-a systematic review and meta-analysis. Curr Neurol Neurosci Rep. 2020; 20:13.

10. Hoh BL, Ko NU, Amin-Hanjani S, Chou SH-Y, Cruz-Flores S, Dangayach NS, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023; 54:e314–70.

11. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.

12. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.

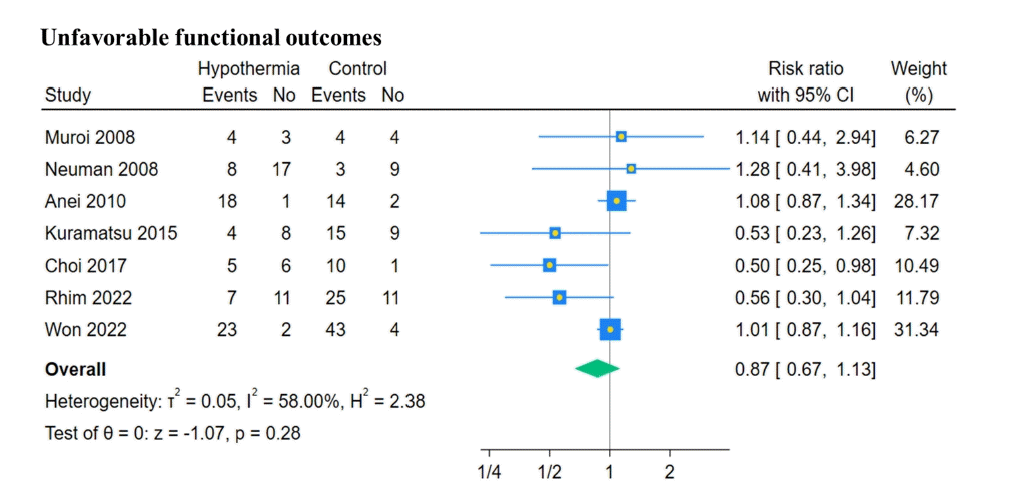
13. Muroi C, Frei K, El Beltagy M, Cesnulis E, Yonekawa Y, Keller E. Combined therapeutic hypothermia and barbiturate coma reduces interleukin-6 in the cerebrospinal fluid after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2008; 20:193–8.

14. Neuman E, Smrčka M, Gál R, Jura R. Mild controlled hypothermia: a neuroprotective method for late ischaemic complications in resuscitation care for patients with severe spontaneous subarachnoid hemorrhage caused by aneurysm rupture. Ceska Slovenska Neurologie Neurochirurgie. 2008; 71:180–7.
15. Anei R, Sakai H, Iihara K, Nagata I. Effectiveness of brain hypothermia treatment in patients with severe subarachnoid hemorrhage: comparisons at a single facility. Neurol Med Chir (Tokyo). 2010; 50:879–83.
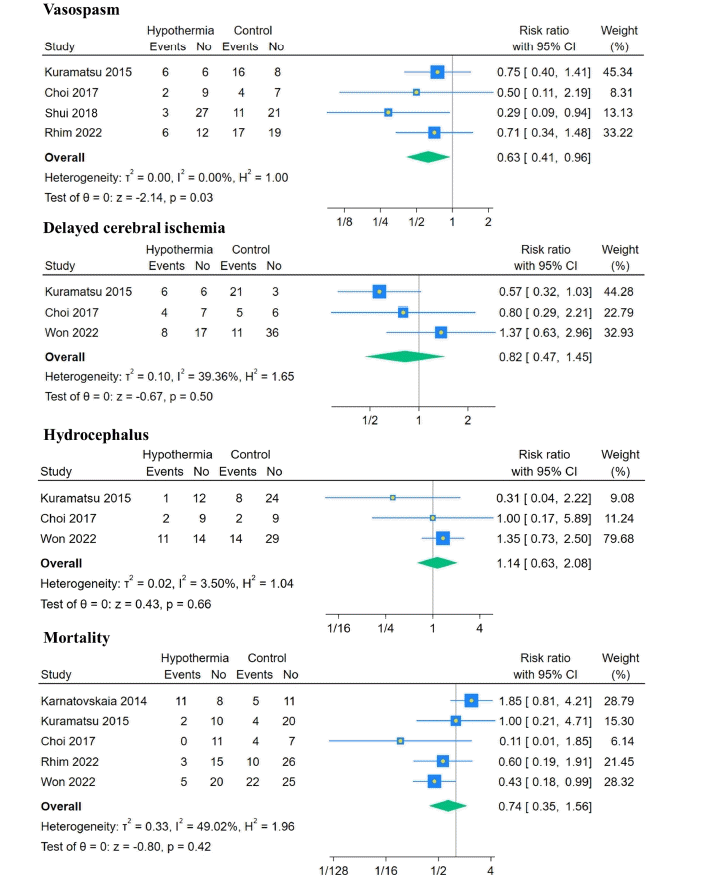
16. Karnatovskaia LV, Lee AS, Festic E, Kramer CL, Freeman WD. Effect of prolonged therapeutic hypothermia on intracranial pressure, organ function, and hospital outcomes among patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2014; 21:451–61.

17. Kuramatsu JB, Kollmar R, Gerner ST, Madžar D, Pisarčíková A, Staykov D, et al. Is hypothermia helpful in severe subarachnoid hemorrhage? An exploratory study on macro vascular spasm, delayed cerebral infarction and functional outcome after prolonged hypothermia. Cerebrovasc Dis. 2015; 40:228–35.

18. Choi W, Kwon SC, Lee WJ, Weon YC, Choi B, Lee H, et al. Feasibility and safety of mild therapeutic hypothermia in poor-grade subarachnoid hemorrhage: prospective pilot study. J Korean Med Sci. 2017; 32:1337–44.

19. Shui T, Guo ZY, Zhang GZ, Chen Q, Li B. Effect and significance of mild hypothermia on cerebral blood flow velocity and cerebral extraction rate of oxygen in patients with severe subarachnoid hemorrhage. Zhonghua Yi Xue Za Zhi. 2018; 98:1489–92.
20. Rhim JK, Park JJ, Kim H, Jeon JP. Early and prolonged mild hypothermia in patients with poor-grade subarachnoid hemorrhage: a pilot study. Ther Hypothermia Temp Manag. 2022; 12:229–34.

21. Won SY, Kim MK, Song J, Lim YC. Therapeutic hypothermia in patients with poor-grade aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2022; 221:107369.

22. Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015; 373:2403–12.

23. Neugebauer H, Schneider H, Bösel J, Hobohm C, Poli S, Kollmar R, et al. Outcomes of hypothermia in addition to decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: a randomized clinical trial. JAMA Neurol. 2019; 76:571–9.

24. Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y, Brain-Hypothermia Study Group. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015; 32:422–9.

25. Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011; 10:131–9.

26. Su Y, Fan L, Zhang Y, Zhang Y, Ye H, Gao D, et al. Improved neurological outcome with mild hypothermia in surviving patients with massive cerebral hemispheric infarction. Stroke. 2016; 47:457–63.

27. Kim JH, Nagy Á, Putzu A, Belletti A, Biondi-Zoccai G, Likhvantsev VV, et al. Therapeutic hypothermia in critically ill patients: a systematic review and meta-analysis of high quality randomized trials. Crit Care Med. 2020; 48:1047–54.

28. Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008; 39:3242–7.

29. Lord AS, Karinja S, Lantigua H, Carpenter A, Schmidt JM, Claassen J, et al. Therapeutic temperature modulation for fever after intracerebral hemorrhage. Neurocrit Care. 2014; 21:200–6.

30. Broessner G, Beer R, Lackner P, Helbok R, Fischer M, Pfausler B, et al. Prophylactic, endovascularly based, long-term normothermia in ICU patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke. 2009; 40:e657–65.
31. Kupchik NL. Development and implementation of a therapeutic hypothermia protocol. Crit Care Med. 2009; 37:S279–84.

32. Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011; 14:389–94.

33. Tan L, Yao D. Meta-analysis for the prognosis of subarachnoid hemorrhage treated with mild hypothermia. Asian J Surg. 2023; 46:5674–6.

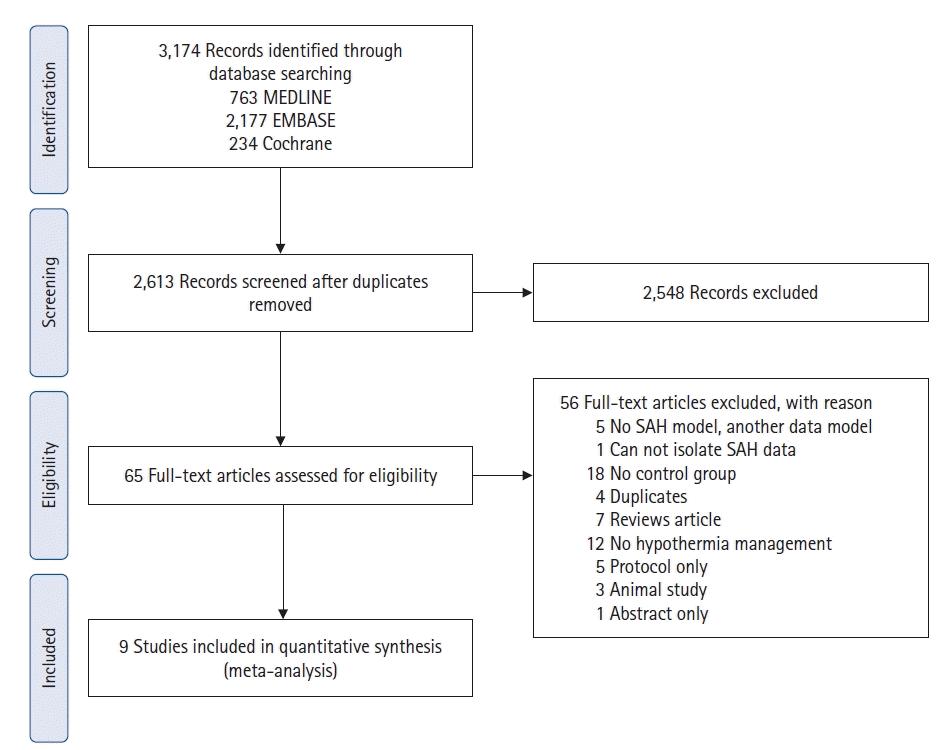
34. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane;2008.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download