See letter "Endoscopic resection penetrating the muscularis propria for gastric gastrointestinal stromal tumors: advances and challenges" in Volume 57 on page 329.
Abstract
Background/Aims
To overcome the technical limitations of classic endoscopic resection for gastric gastrointestinal stromal tumors (GISTs), various methods have been developed. In this study, we examined the role and feasibility of clip-and-cut procedures (clip-and-cut endoscopic full-thickness resection [cc-EFTR]) for gastric GISTs.
Methods
Medical records of 83 patients diagnosed with GISTs after endoscopic resection between 2005 and 2021 were retrospectively reviewed. Moreover, clinical characteristics and outcomes were analyzed.
Results
Endoscopic submucosal dissection (ESD) and cc-EFTR were performed in 51 and 32 patients, respectively. The GISTs were detected in the upper third of the stomach for ESD (52.9%) and cc-EFTR (90.6%). Within the cc-EFTR group, a majority of GISTs were located in the deep muscularis propria or serosal layer, accounting for 96.9%, as opposed to those in the ESD group (45.1%). The R0 resection rates were 51.0% and 84.4% in the ESD and cc-EFTR groups, respectively. Seven (8.4%) patients required surgical treatment (six patients underwent ESD and one underwent cc-EFTR,) due to residual tumor (n=5) and post-procedure adverse events (n=2). Patients undergoing R0 or R1 resection did not experience recurrence during a median 14-month follow-up period, except for one patient in the ESD group.
Current clinical guidelines recommend periodic endoscopic surveillance for subepithelial tumors (SETs) <20 mm due to their benign features, while surgical intervention is preferred for large SET lesions.1-4 However, certain small SETs have malignant potential, especially gastrointestinal stromal tumors (GISTs).5 Approximately 3.7% of benign-looking GISTs <20 mm in size have a high mitotic index and rapidly increase in size.6 Therefore, for small SETs, endoscopic follow-up may lead to either missed or delayed diagnosis of malignancy. Moreover, periodic endoscopic checkups can be stressful and troublesome for patients. Therefore, when SETs suspected to be GISTs are identified using various diagnostic tools, endoscopic resection (ER) is an option for definitive histopathological diagnosis, as well as complete removal.
For localized gastric GISTs, surgical resection (SR) is the treatment of choice.7,8 With the advancements in ER techniques and the introduction of procedures, such as endoscopic submucosal dissection (ESD), submucosal tunneling endoscopic resection (STER), and endoscopic full-thickness resection (EFTR), numerous attempts to treat gastric GISTs endoscopically have been made. In a recent study conducted at our center, ER was proven to be an effective and comparable treatment choice to SR for removing gastric GISTs <5 cm.9 In this study, two forms of ER were used: a standard ESD approach and a modified EFTR method used to cater to the procedure’s limitations. We devised a modified EFTR called “clip-assisted EFTR (cc-EFTR)” to facilitate the removal of gastric SETs located in the deep layer, similar to the EFTR technique. Furthermore, cc-EFTR was used for the treatment of areas that were difficult to access or resect with traditional ESD. This study evaluated the feasibility of ER and compared the clinical outcomes of ESD and cc-EFTR to determine the efficacy of the cc-EFTR method.
This single-center, retrospective longitudinal cohort study included patients who underwent ER therapy for gastric GISTs at the Asan Medical Center, Seoul, Korea. Between June 2005 and December 2021, 104 patients underwent ER for SETs <5 cm in size, which were confirmed to be GISTs by clinical pathologists. During the same period, 1,011 patients underwent therapeutic procedures for gastric SETs, of which 637 patients received surgical treatment and 270 patients received ERs for SETs other than GISTs. In our center, follow-up was preferentially performed in cases of gastric SETs with an initial size of ≤25 mm. Additionally, ER was attempted if the SET was suspected to be a gastric GIST, a change in size or shape was observed, or the patient strongly wanted ER instead of surgical removal. Patients with clinicopathological data that could not be graded according to the modified 2008 National Institute of Health (NIH) consensus criteria,7,10 those who underwent ER techniques other than ESD or cc-EFTR, those who had no pre-treatment endoscopic ultrasonography (EUS) data, and those with GISTs >25 mm on initial EUS imaging were excluded. Thus, the data of 83 patients were analyzed. The flowchart of patient inclusion is displayed in Figure 1.
All patients’ clinical and pathological data were obtained and analyzed from medical records, including endoscopic and EUS images, final pathological reports, endoscopy record sheets, operation reports, hospital stay, and individual information. Moreover, EUS was utilized to evaluate the characteristics of SETs, including the initial and final size (last measured size before the procedure), echogenicity and homogeneity, the presence or absence of cystic foci and hyperechoic foci, which are associated with the malignant nature of GISTs.11,12 All eligible patients underwent esophagogastroduodenoscopy (EGD) and EUS before treatment. The type of ER procedure chosen depended on the endoscopists’ decision. Risk stratification of gastric GIST was evaluated according to the modified NIH consensus criteria.7,10
Until 2014, ESD was mainly performed for gastric SETs. However, after the development of cc-EFTR in 2014, endoscopists exercised discretion in selecting the treatment method, opting for either ESD or cc-EFTR. The choice was based on the location and characteristics of the lesion. All procedures were performed under conscious sedation with intravenous administration of 0.05 to 0.1 mg/kg of midazolam or 0.5 to 1 mg/kg of propofol, and 1.5 L/min of room air was insufflated through the endoscope. We used normal saline mixed with indigo carmine and a small amount of 0.005% epinephrine to lift the submucosal space of the lesion. A transparent cap (D-201-11814; Olympus) was applied at the tip of the forward-viewing single-channel endoscope (GIF-H260 or GIF-HQ290; Olympus). Before the ER, we observed the lesion using white-light endoscopy and narrow-band imaging to determine the location. Insulated-tip knife-2 (KD-611L; Olympus), dual knife J (KD-655L; Olympus), and/or hook knife (KD-620LR; Olympus) were used to remove the SETs. Hemostatic clips (HX-610; Olympus) or RAICHO2 forceps (RC1550-2WE; Kaneka Medix) were used to control bleeding during the procedure.
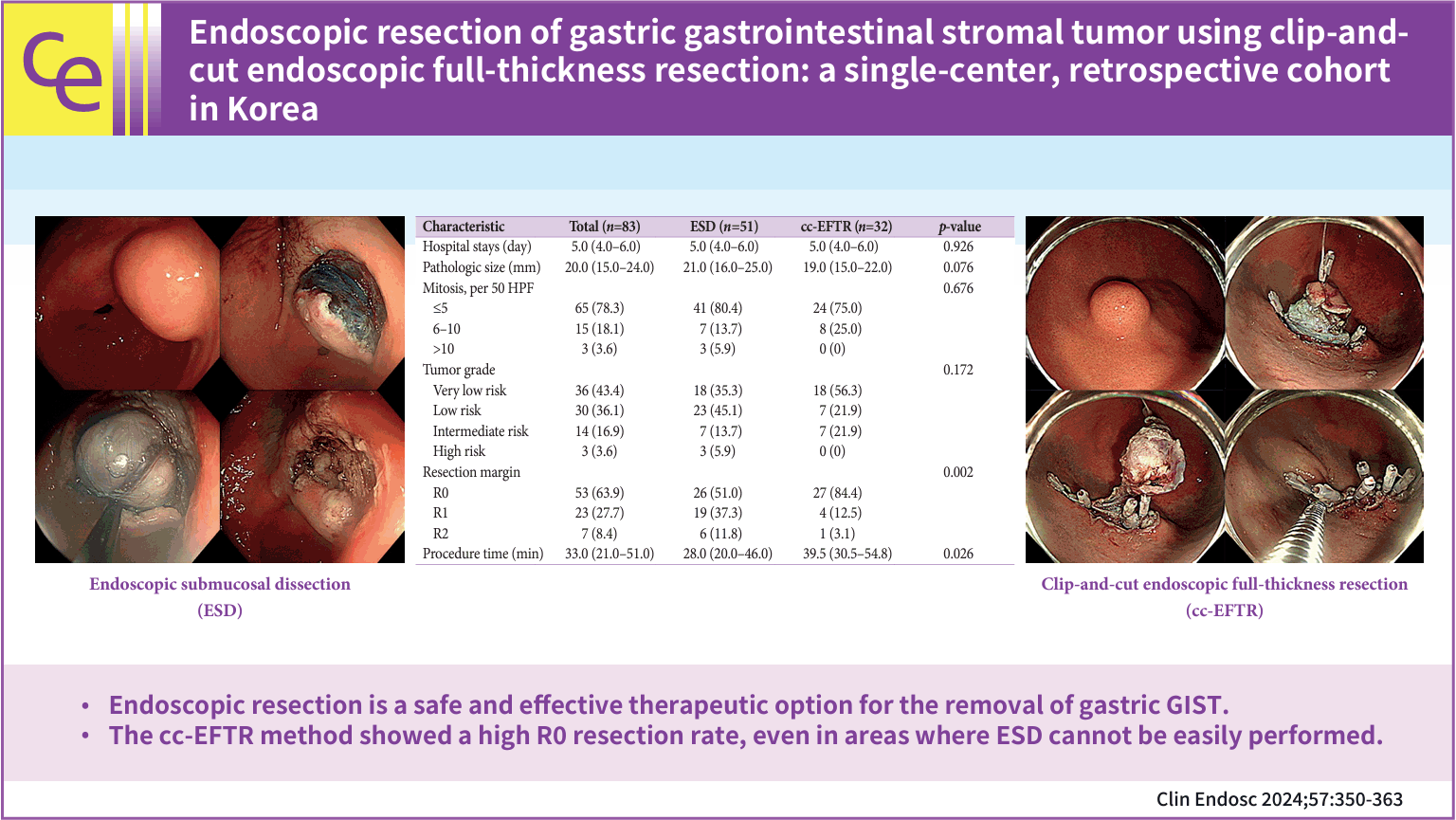
ESD was performed following the conventional technique: lesion border marking, submucosal injection, circumferential incision, and submucosal dissection (Fig. 2A–H). In the cc-EFTR method, following marking and submucosal injection, the operator attached a clip with dental floss to the edge of the dissected flap (Fig. 2I–P). The operator next retrieved the dental floss with the endoscope and reinserted the endoscope into the stomach while tugging with sufficient force to enable adequate visualization of the submucosal space and to prevent tearing the mucosal layer of the flap. In the process of partially dissecting the submucosal layer and identifying the muscularis propria (MP) layer that contained the SET, the operator made a small incision in the MP layer and approached the tumor. Before dissecting the MP layer, two to four sentinel clips were placed at both ends of the excision site to anchor the MP layer and prevent the transmural hole from enlarging. After meticulously dissecting the submucosal layer, the operator made a crescent-shaped transmural incision following the direction, in which each sentinel clip was attached, and evaluated the intra-abdominal structures by pulling the traction to ensure the transmural dissection was done. Transmural excision was performed gradually while continually dragging the traction and SET into the gastric cavity, and clips were used to preventatively suture the dissected area. This preventive clipping just after the transmural cut was repeated stepwise throughout the procedure until the entire dissected section was completely sutured. Then, the SET was captured inside the stomach, except for a very narrow connection with the MP layer. Once the perforated MP layer was adequately sutured, the final attachment to the remaining muscle layer was severed. After additional clipping at the excision site to confirm that the muscle layer was securely sutured, the specimen was retrieved, and the procedure was completed (Supplementary Video 1).
The specimens were processed with hematoxylin and eosin staining for evaluation. Immunohistochemical (IHC) staining for cluster of differentiation (CD) 117 (c-kit), CD34, desmin, smooth muscle actin, Ki-67, and S-100 was employed. Positive results in each IHC staining were regarded as confirmation of GIST. The tumor size and mitotic count per 50 high-power fields (HPFs) of each specimen were evaluated by clinical pathologists. These results were used for risk stratification of gastric GIST based on the modified NIH consensus criteria.7,10
Chest radiographs were performed on all the patients to assess for adverse events, such as pneumothorax and pneumoperitoneum after the procedure. On hospitalization day 2 or 3, a follow-up EGD was performed. Patients who did not experience post-ER adverse events were discharged as their diet progressed. Proton pump inhibitors were administered for 2 to 4 weeks after the procedure to prevent delayed bleeding. EGD and abdominal computed tomography (CT) were performed annually for 5 years.
The location of gastric GISTs was classified into upper, middle, and lower parts of the stomach. The upper part included the fundus, cardia, and high body; the middle part included the mid and lower body; and the lower part included the gastric angle and antral portion. The resection margins were evaluated by clinical pathologists. We divided the SETs according to the extent of MP layer involvement (e.g., none, <50%; involved ≥50%; and outside of MP), as evaluated by the EUS performed by expert endoscopists. An R0 resection was defined as no gross or microscopic tumor remaining in the primary tumor bed with a negative resection margin. Complete resection was referred to as R0 resection. An R1 resection was defined when remnant tumor cells were observed microscopically on the resection margin. Additionally, an R2 resection was defined as gross remnant tumors. Peritonitis was defined as a case accompanied by both rebound tenderness of the abdominal wall in physical examination and the presence of fever (≥38 ℃ of the body temperature) after the procedure. The procedure time was defined as the duration between the beginning of marking and the extraction of the SETs through the mouth.
Baseline characteristics between the ESD and cc-EFTR groups were compared. Quantitative data, such as age, tumor size, procedure time, and hospital stay, were expressed as medians with interquartile ranges (IQRs). Qualitative data, such as sex, symptoms, location, shape, pathological grade of tumor, and resection rate were expressed as proportions. Categorial variables were compared in non-parametric tests using the Kruskal–Wallis test. A probability level (p) of 0.05 was chosen for statistical significance. Univariate and multivariate analyses were performed in addition to logistic regression analysis with backward elimination. All statistical analyses were performed using IBM SPSS Statistics for Windows ver. 21.0 (IBM Corp.) and Prism 9 for Windows ver. 9.3.1 (GraphPad Software Inc.).
The baseline patient characteristics are displayed in Table 1. Among 83 patients who underwent therapeutic ER procedures for gastric GIST, ESD and cc-EFTR were performed in 51 (61.4%) and 32 (38.6%) patients, respectively. The incidence of gastric GISTs was high in the upper body (52.9% for ESD vs. 90.6% for cc-EFTR, p<0.001). Compared to the cc-EFTR, the number of GISTs containing cystic foci was higher in the ESD group (96.1% for ESD vs. 81.3% for cc-EFTR, p=0.027). In comparison to the ESD group, gastric GISTs treated with cc-EFTR exhibited a higher percentage of lesions situated outside of the MP layer (23.5% for ESD vs. 71.9% for cc-EFTR, p<0.001). Additionally, the shapes of these lesions were less protruding (52.9% for ESD vs. 34.4% for cc-EFTR, p=0.035).
The endoscopic results of each method are described in Table 2. Overall, 79.5% of gastric GIST demonstrated very low and low-risk tumor grades, and the overall R0 and R1 resection rate was 91.6%. To determine the differences based on the location, in treatment outcomes of each method, we divided the location of the lesion into two categories: upper third, and the middle plus lower third (Fig. 3). In the upper third, the cc-EFTR group exhibited a higher R0 resection rate than the ESD group (44.4% for ESD vs. 86.3% for cc-EFTR, p=0.001) without a significant difference in the procedure time (28.0 minutes for ESD vs. 39.0 minutes for cc-EFTR, p=0.109). However, in the middle plus lower third, neither the resection margin (58.3% for ESD vs. 66.7% for cc-EFTR, p=0.437) nor the procedure time (27.0 minutes for ESD vs. 40.0 minutes for cc-EFTR, p=0.449) displayed any significant differences between two methods. During univariate and multivariable analysis associated with R0 resection, ER with cc-EFTR method (odds ratio [OR], 5.192; 95% confidential interval [CI], 1.727–15.613; p=0.03) was discovered to be an independent predictive factor of R0 resection (Table 3). Five patients in the ESD group underwent additional staged surgical treatments after the ERs. The patients underwent additional SR due to R2 resection (four patients with wedge resection and one patient with total gastrectomy). The summary of patients who underwent staged additional surgeries after ERs are presented in Table 4.
A total of 43 (51.8%) patients experienced adverse events after ER (Table 5). The overall rates of adverse events were higher in the cc-EFTR group compared to that in the ESD group (37.3% for ESD vs. 75.0% for cc-EFTR, p=0.001). Pneumoperitoneum occurred in 13 (25.5%) and 22 (68.8%) patients in the ESD and cc-EFTR groups, respectively. However, only one patient in the ESD group and two in the cc-EFTR group progressed to localized peritonitis, however, they recovered after conservative care, including broad-spectrum antibiotic administration (2.0% for ESD vs. 6.3% for cc-EFTR, p=0.311). Two patients underwent emergent operations during the ER. In the ESD group, a patient with severe bleeding during the procedure underwent emergent wedge resection. In the cc-EFTR group, a patient with failed endoscopic closure for a huge transmural hole during ER underwent emergent wedge resection. All other patients were managed with conservative treatment, including endoscopic closure with hemostatic clips and intravenous antibiotics, without any further adverse events. A summary of patients who underwent surgical procedures after ERs due to endoscopic adverse events is displayed in Table 4.
The median follow-up period for all patients was 25.0 months. One patient in the ESD group experienced GIST recurrence. A 77-year-old man with underlying metastatic prostate cancer was incidentally diagnosed with a 22 mm-sized GIST in the lower body during his routine EGD examination. After 4-year follow-up, the SET increased in size to 26 mm, leading to the decision to undergo ESD. The pathological results displayed an intermediate risk for gastric GIST according to the modified NIH criteria. The resection margin was clear in the final clinicopathological report, and his serial follow-up EGD displayed no residual tumor. After 4 years of ER, a 28 mm-sized recurrent exophytic GIST was observed in the high body of the stomach on follow-up CT images. Considering the patient’s age and performance status, the patient was observed without additional ER or SR. The patient died 17 months after diagnosis of a recurrent tumor due to the progression of prostate cancer.
One patient in the cc-EFTR group received adjuvant chemotherapy with the tyrosine kinase inhibitor, imatinib. A 75-year-old man underwent cc-EFTR for a 19.2 mm-sized gastric GIST with a mitotic count of 10/50 HPFs; therefore, he was categorized as an intermediate-risk group patient according to the modified NIH criteria. However, as the mitotic count was 10/50 HPF and the risk of recurrence was relatively high in the groups with indeterminate risk, adjuvant imatinib chemotherapy was initiated after consultation with the oncologist. After 5 days, he experienced angioedema as a side effect of imatinib, due to which he stopped the chemotherapy. Subsequent follow-ups through the outpatient clinic displayed no evidence of recurrence. These results are summarized in Figure 4.
The procedure of cc-EFTR was developed to overcome the limitations of EFTR, reduce the size of muscle defect at the time of closure, and reduce the procedure time. In our study, we compared the efficacy of ER and the safety of the cc-EFTR and ESD methods in removing small gastric GISTs. We discovered that ER demonstrated R0 and R1 resection rates of 63.9% and 27.7%, respectively, and during the 25 months follow-up period, only one patient experienced recurrence after ER. Specifically, cc-EFTR demonstrated a significantly high R0 resection rate, especially in the upper part such as the fundus, cardia, and high body. Despite the high incidence of adverse events compared to the ESD group, all but one patient, who underwent emergent surgery during the procedure, were managed with conservative care, indicating that the cc-EFTR method can be one of the possible options for removing gastric GISTs.
Although SR is the standard treatment for gastric GISTs in the National Comprehensive Cancer Network guideline and endoscopic treatment is not widely recommended owing to the lack of long-term safety, several studies have suggested ER as an alternative therapeutic option. Our previous study compared the safety and efficacy of ER and SR for small gastric GISTs <5 cm. Statistically, the R0 resection rate for ER was 60.8%, which was lower than that for SR (98.5%); nevertheless, no tumor recurrence was observed during the 47.9 months follow-up period.9 Joo et al.13 also evaluated the feasibility and long-term outcomes of ER against SR. In the ER group, tumor size was smaller and the R0 resection rate was lower compared to that in the SR group (25.0% vs. 85.0%). However, no significant difference in the recurrence rate was observed during the 45.5 months follow-up period, consistent with our findings.13 In this study, the R0 resection rate was 63.9% and confirmed as 84.4% in the cc-EFTR group, equivalent to the R0 resection rate in the surgical group confirmed in the prior studies.
Although the R0 resection rate of ER was lower than that of SR in previous reports, no patients undergoing R1 and R2 resection in the cc-EFTR group experienced recurrence. Unlike those undergoing R2 resection, the majority of those undergoing R1 resection did not receive surgery, and despite the follow-up observation, no recurrence or metastasis was observed. R1 resection may not indicate an incomplete excision; rather, it could be a result according to the three-dimensional characteristics of GISTs. GISTs are enveloped by a pseudocapsule and necessitate en bloc enucleation to prevent tumor spillage. In contrast to flat lesions, such as adenomas or cancers, the pathological evaluation of the resection margin of GISTs with a three-dimensional structure surrounded by pseudocapsules might be difficult to evaluate precisely because of tissue deformation during sectioning and chemical fixation. Therefore, R1 resection does not always indicate incomplete resection owing to the possibility of false-positive margins.9 Moreover, en bloc resections were performed in all 83 procedures of the present study; thus, the potential for post-procedural adverse events, such as peritoneal seeding, was minimal or negligible. Several studies have investigated the impact of R1 resection on the prognosis of gastric GISTs, including the recurrence of the GISTs. A study conducted by McCarter et al.14 in 2012 revealed that 72 (8.8%) out of the 819 patients who underwent SR of gastrointestinal GISTs had an R1 resection status. Moreover, no significant differences were observed in recurrence-free survival between the R0 and R1 groups, regardless of the administration of adjuvant imatinib (p=0.73) or placebo (p=0.24). Additionally, a study by Joo et al.13 in 2016 involved the endoscopic removal of GISTs in the upper gastrointestinal (GI) tract in 90 patients, among whom pathological R1 resection was confirmed in 65 individuals (72.2%). Throughout a median follow-up period of 45.5 months, only two patients in the R1 resection group experienced recurrence. Furthermore, a study by Zhu et al.15 in 2020 analyzed the relationship between the status of the resection margin and the recurrence rate of gastrointestinal mesenchymal tumors (GIMTs), including GISTs in the stomach.15 The R1 resection group of gastric GIMTs did not experience a higher recurrence rate than the R0 resection group (p=0.84).
In terms of adverse events, more patients had post-procedure fever and pneumoperitoneum after undergoing cc-EFTR than those undergoing ESD. Although room air, not carbon dioxide gas, was used throughout the endoscopic procedure and the procedure was performed under conscious sedation, not under general anesthesia, no changes in vital signs accompanied pneumoperitoneum during or after the procedure. This observation is similar to that of postoperative pneumoperitoneum, which is a benign condition that spontaneously remits and is seen as an unavoidable consequence of transmural excision of deeply seated tumors.16 Only one patient underwent emergent surgery immediately after the cc-EFTR procedure. The site that underwent transmural resection was left without clip closure due to challenges in positioning. This area was specifically the junction between the greater curvature and the posterior wall side of the upper body. Except for one patient who underwent surgical conversion during cc-EFTR, all patients were successfully treated primarily using endoscopic clips, and none of the patients with pneumoperitoneum in post-procedural radiographs progressed to life-threatening peritonitis.
In our study, GISTs were mainly removed using ESD or cc-EFTR, and the complete resection rate was significantly higher in the cc-EFTR group than that in the ESD group. Additionally, ESD can be an effective ER method, but the applicability of the procedure is limited as it is beneficial mainly on SETs with narrow connections with the MP layer.17-19 Białek et al.19 retrospectively analyzed the clinical outcomes of ESD for 37 patients with SETs, including 17 (46%) with gastric GISTs, and a difference in the complete resection rate was observed according to the connection with the MP (100% in SETs with no connection vs. 68.2% in SETs with connection). An et al.18 also evaluated the feasibility of ESD in removing gastric GISTs, as well as the risk factor of a gastric wall defect. The risk of perforation was high when the surface connecting to the MP was wide or the lesion extended below the MP layer. In our data, 28 (54.9%) tumors demonstrated none or <50% MP involvement, and 27 (52.9%) tumors had a pedunculated shape in the ESD group, indicating that the technique was more often performed for superficially located lesions. In the cc-EFTR group, however, 31 (96.9%) tumors had ≥50% MP involvement and the morphology of the SETs were not confined, suggesting cc-EFTR may be beneficial for gastric GISTs with a wide range of shape and location. Moreover, the R0 resection rate was high in lesions located in the upper third. This is considered clinically significant, as determining the exact shape and location of gastric GISTs using EUS alone is difficult. The procedural difficulty, which is thought to be associated with the location and depth of the SETs, may be relieved by continuously drawing the SETs into the gastric cavity using a clip, where dental floss is attached throughout the process.
In conventional EFTR, wall repair with a suture/clip device or laparoscope may be necessary if the size of the stomach wall perforation generated after the transmural dissection of the entire gastric layers is too large to be managed by hemoclips.20,21 In this study, we used the hemostatic clips to seal the hole during every small transmural cut, which is readily available in most situations. Universality is an advantage when using a clip to seal a hole as it is easy to use, can be utilized at any moment during the procedure, does not require additional device installation, and can be utilized in the majority of endoscopic centers. Various novel devices, such as over-the-scope clips (OTSCs) and endoscopic suturing devices were developed and utilized in removing gastric SETs.22-25 Although these devices were designed to securely and easily close the transmural perforation site, they are only used in a limited number of endoscopic centers, and their high cost is a drawback. In OTSC, transmural resection of SETs, as well as closing perforation site is available. However, as the procedure with OTSC is conducted without visually checking the deep resection margin, a danger persists of remnant tumors after resection. Throughout the simple repetition of closing processes with the clips, the cc-EFTR displayed advantages in an easy approximation of the resected lesion and non-inferior results in the procedure time compared to ESD.
Our study only investigated ESD or cc-EFTR, not STER, as the number of clinical situations where STER could be applied was quite limited. Furthermore, STER has been used as a good option for removing SETs as the procedure can promote rapid wound healing and further decrease adverse event rates, ever since the first clinical report by Inoue et al.26 A systemic review and meta-analysis also reported both high complete (97.5%) and en bloc (94.6%) resection rates for STER.27 However, STER may be difficult to perform clinically in many instances, hence the number of cases in which STER was performed in our institution was extremely low and excluded from the study. During the STER procedure, the operator must form a submucosal tunnel using an endoscopic knife to remove gastric GISTs located in the deep MP layer; however, procedure time increased in this process. Additionally, laceration or perforation of the gastric mucosal layer can occur during submucosal tunnel formation in patients with a moderate to severe degree of atrophic change in the gastric mucosa. Moreover, the application of STER to lesions situated in challenging areas of the stomach, where forming a tunnel is difficult due to the polygonal shape, such as the gastric fundus and lesser curvature, poses a challenge. This difficulty could potentially result in serious adverse events.
This study had certain limitations. First, as this was a retrospective, observational, and single-center study, the results may have been limited. Given these points, the patient population might be limited; furthermore, the decision to introduce an endoscopic treatment regimen of gastric SETs was based on the physician’s discretion, which might have introduced bias. Additionally, as the cc-EFTR method was devised in 2014, the follow-up duration may be short. Thirdly, because endoscopic resection of gastric SET is challenging for novice therapeutic endoscopists and requires sufficient experience with endoscopic procedures to competently perform, the risks of the procedure may have been relatively understated. Furthermore, even though the price is less than that of other suture devices, such as OTSCs, additional expenses may be incurred when using clips or dental floss. Finally, the cc-EFTR group required a longer procedure duration than the ESD group. To accurately evaluate the duration of each procedure, lesions in comparable locations should be compared. Nevertheless, in this study, the preferred endoscopic treatment varied depending on the location of the lesion, which might have affected the study findings. Therefore, considering the outcomes of this study, a future multi-center, long-term follow-up prospective study should be conducted to evaluate the efficacy of cc-EFTR more comprehensively. Despite these limitations, our study results suggest that ER is a safe and feasible method for gastric SETs and that the cc-EFTR method was feasible for GISTs in terms of curability and safety, especially when located in the fundus, cardia, and high body.
In conclusion, ER seems to be a safe and effective therapeutic option for the removal of gastric GISTs. The cc-EFTR method in removing GIST transmurally exhibited a higher R0 resection rate, which was useful and safe for complete resection, even in areas where ESD cannot be easily performed.
Supplementary Material
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2023.144.
Notes
Conflicts of Interest
Supplementary Video 1 included in this paper is a partially modified version of a case that was presented at the DDW’s video forum in 2017. Ji Yong Ahn is currently serving as a section editor for Clinical Endoscopy; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. The other authors have no potential conflicts of interest.
REFERENCES
1. Deprez PH, Moons LM, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022; 54:412–429.

2. Lu J, Lu X, Jiao T, et al. Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol. 2014; 48:667–673.

3. Cho JW; Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:235–240.

4. Yang Z, Gao Y, Fan X, et al. A multivariate prediction model for high malignancy potential gastric GI stromal tumors before endoscopic resection. Gastrointest Endosc. 2020; 91:813–822.

5. Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019; 10:144–154.

6. Ko WJ, Cho JY. Current techniques for treating gastrointestinal stromal tumors in the upper gastrointestinal tract. Clin Endosc. 2016; 49:226–228.

7. Schmieder M, Henne-Bruns D, Mayer B, et al. Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resection. Front Pharmacol. 2016; 7:504.

8. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8 Suppl 2(0 2):S1–S41.

9. Kim GH, Choi KD, Gong CS, et al. Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score-matched case-control study. Gastrointest Endosc. 2020; 91:527–536.

10. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.

11. Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997; 45:468–473.

12. Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000; 46:88–92.

13. Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016; 83:318–326.

14. McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012; 215:53–60.

15. Zhu Y, Xu MD, Xu C, et al. Microscopic positive tumor margin does not increase the rate of recurrence in endoscopic resected gastric mesenchymal tumors compared to negative tumor margin. Surg Endosc. 2020; 34:159–169.

16. Chapman BC, McIntosh KE, Jones EL, et al. Postoperative pneumoperitoneum: is it normal or pathologic? J Surg Res. 2015; 197:107–111.

17. Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012; 75:1153–1158.

18. An W, Sun PB, Gao J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017; 31:4522–4531.

19. Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012; 75:276–286.

20. Tan Y, Tan L, Lu J, et al. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017; 2:115.

21. Huberty V, Verset L, Deviere J. Endoscopic full-thickness resection of a gastric GI stromal tumor. VideoGIE. 2019; 4:120–122.

22. Guo JT, Zhang JJ, Wu YF, et al. Endoscopic full-thickness resection using an over-the-scope device: a prospective study. World J Gastroenterol. 2021; 27:725–736.

23. Schlag C, Wilhelm D, von Delius S, et al. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013; 45:4–11.

24. Meier B, Schmidt A, Glaser N, et al. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surg Endosc. 2020; 34:853–860.

25. Schmidt A, Bauder M, Riecken B, et al. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015; 47:154–158.

Fig. 1.
Flowchart of patient inclusion. SET, subepithelial tumor; ER, endoscopic resection; GIST, gastrointestinal stromal tumor; NIH, National Institute of Health; STER, submucosal tunneling endoscopic resection; EUS, endoscopic ultrasonography; ESD, endoscopic submucosal dissection; cc-EFTR, clip-and-cut endoscopic full-thickness resection.
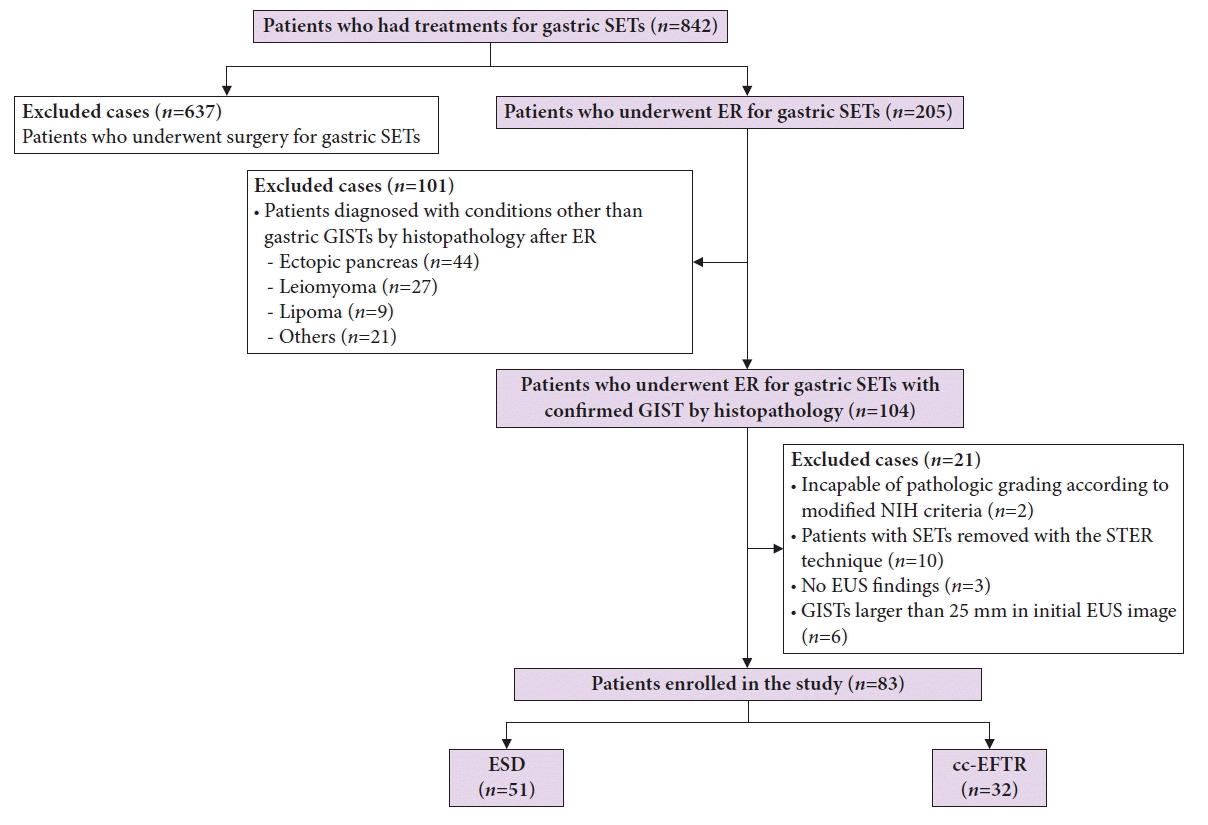
Fig. 2.
(A–H) Case illustration of endoscopic submucosal dissection. (A) A subepithelial tumor (SET) in the distal antrum. (B) After marking around the lesion with an endoscopic knife, submucosal injection is administered. (C–F) Circumferential incision followed by submucosal dissection is performed. (G) After removing the SET, hemostasis is performed with coagulation forceps. (H) The removed gastric SET is displayed. (I–P) Case illustration of clip-and-cut endoscopic full-thickness resection. (I) A SET in the fundus is displayed. (J) Submucosal injection and circumferential incision are performed. (K) A clip with dental floss is applied to the mucosa above the SET for traction. (L) Sentinel clips are placed on both sides of the resected area to anchor the muscularis propria layer. (M) Transmural resection is performed using continuous traction to pull the SET into the stomach. (N) Transmural resection and perforation closure with clips are carried out simultaneously. (O) After the SET is completely excised, additional clipping is performed to strengthen the closure site. (P) The removed gastric SET is displayed.
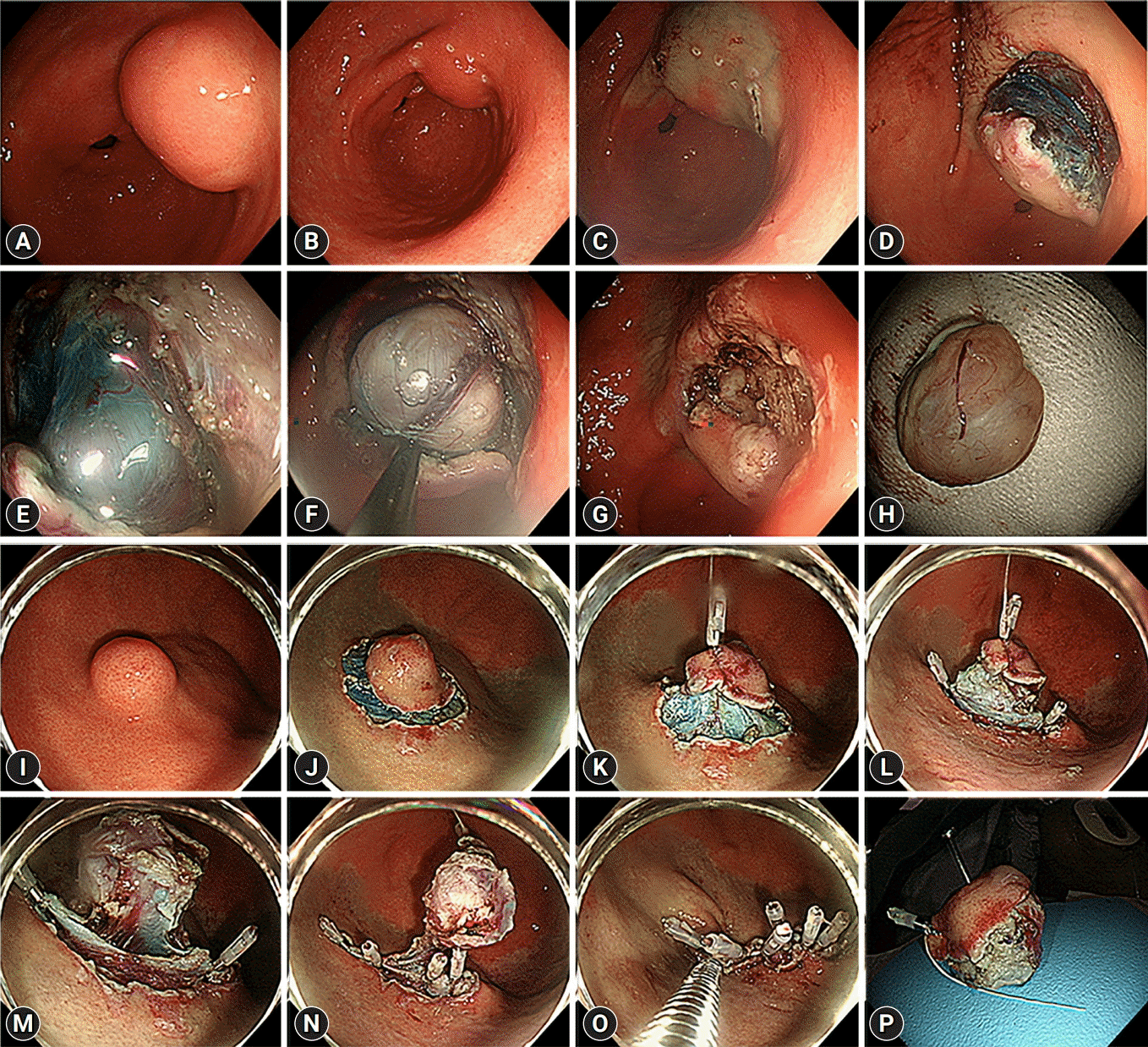
Fig. 3.
Differences between the resection margin status and procedure time according to location. To investigate the efficacy of endoscopic submucosal dissection (ESD) and clip-and-cut endoscopic full-thickness resection (cc-EFTR) according to location, the state of resection margin and procedure time are compared between the upper third and middle plus lower third groups. Unlike the middle plus lower third group, which had no differences in resection margin and procedure time, the R0 resection rate is significantly high in cc-EFTR in the upper third groups. (A, B) Resection margin status of upper third and middle plus lower third. (C, D) Procedure time of upper third and middle plus lower third.
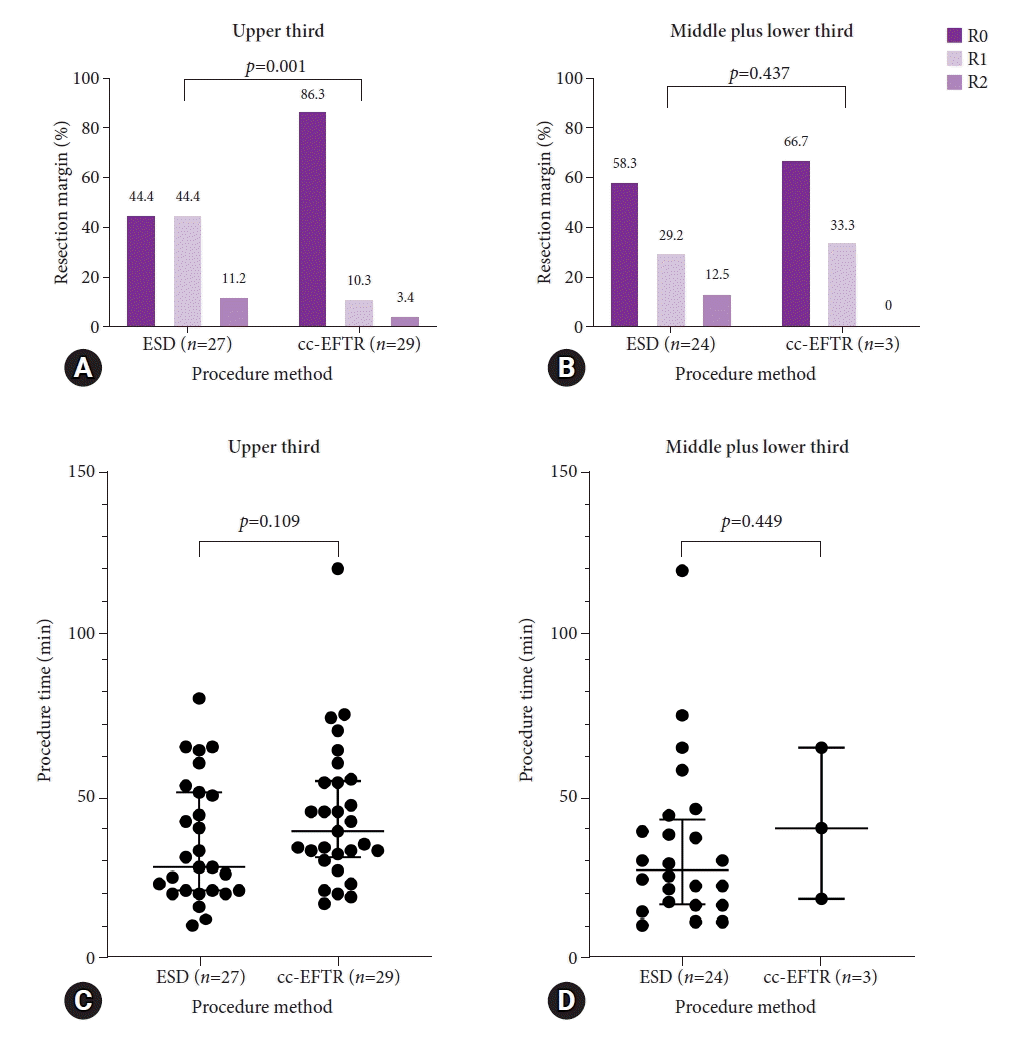
Fig. 4.
Clinical outcomes of the endoscopic resection methods. Summary of each treatment’s oncologic follow-up. ESD, endoscopic submucosal dissection; cc-EFTR, clip-and-cut endoscopic full-thickness resection.
Table 1.
Baseline characteristics of patients and gastric GISTs
Table 2.
Clinical outcomes of the ER methods
| Characteristic | Total (n=83) | ESD (n=51) | cc-EFTR (n=32) | p-value |
|---|---|---|---|---|
| Hospital stays (day) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.926 |
| Pathologic size (mm) | 20.0 (15.0–24.0) | 21.0 (16.0–25.0) | 19.0 (15.0–22.0) | 0.076 |
| Mitosis, per 50 HPF | 0.676 | |||
| ≤5 | 65 (78.3) | 41 (80.4) | 24 (75.0) | |
| 6–10 | 15 (18.1) | 7 (13.7) | 8 (25.0) | |
| >10 | 3 (3.6) | 3 (5.9) | 0 (0) | |
| Tumor gradea) | 0.172 | |||
| Very low risk | 36 (43.4) | 18 (35.3 | 18 (56.3) | |
| Low risk | 30 (36.1) | 23 (45.1) | 7 (21.9) | |
| Intermediate risk | 14 (16.9) | 7 (13.7) | 7 (21.9) | |
| High risk | 3 (3.6) | 3 (5.9) | 0 (0) | |
| Resection margin | 0.002 | |||
| R0 | 53 (63.9) | 26 (51.0) | 27 (84.4) | |
| R1 | 23 (27.7) | 19 (37.3) | 4 (12.5) | |
| R2 | 7 (8.4) | 6 (11.8) | 1 (3.1) | |
| Procedure time (min) | 33.0 (21.0–51.0) | 28.0 (20.0–46.0) | 39.5 (30.5–54.8) | 0.026 |
Table 3.
Univariate and multivariable analysis associated with R0 resection
Table 4.
Summary of patients who underwent surgical procedures after ER




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download