INTRODUCTION
METHODS
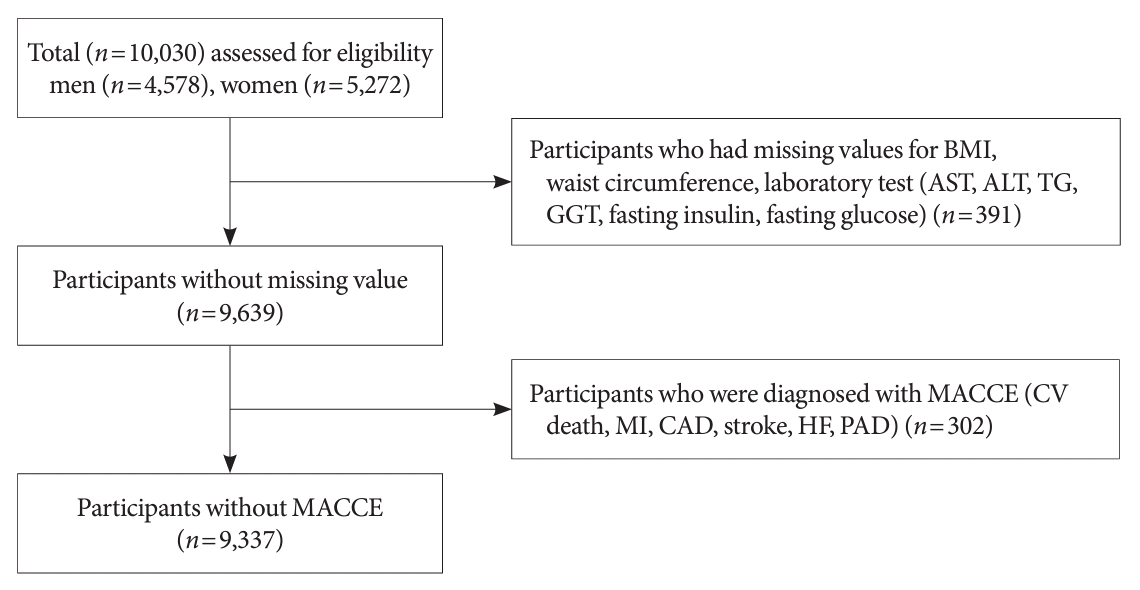
Study population
Fig. 1.
Data collection
Study outcomes
Metabolic index calculation
Statistical analyses
RESULTS
Baseline characteristics
Table 1.
Values are presented as mean±standard deviation or number (%).
IQR, interquartile range; METS, metabolic score; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; TyG, triglyceride glucose index; HOMA-IR, homeostatic model assessment for insulin resistance; METS-IR, metabolic score for insulin resistance.
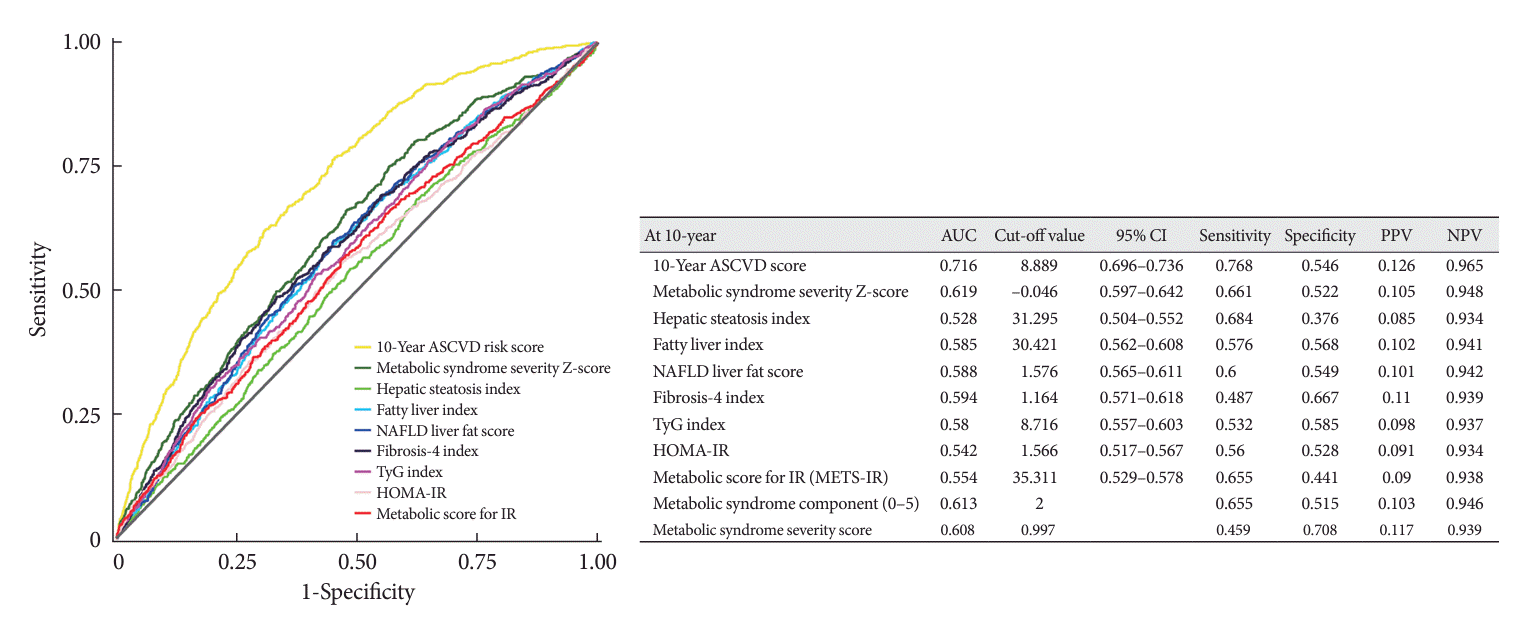
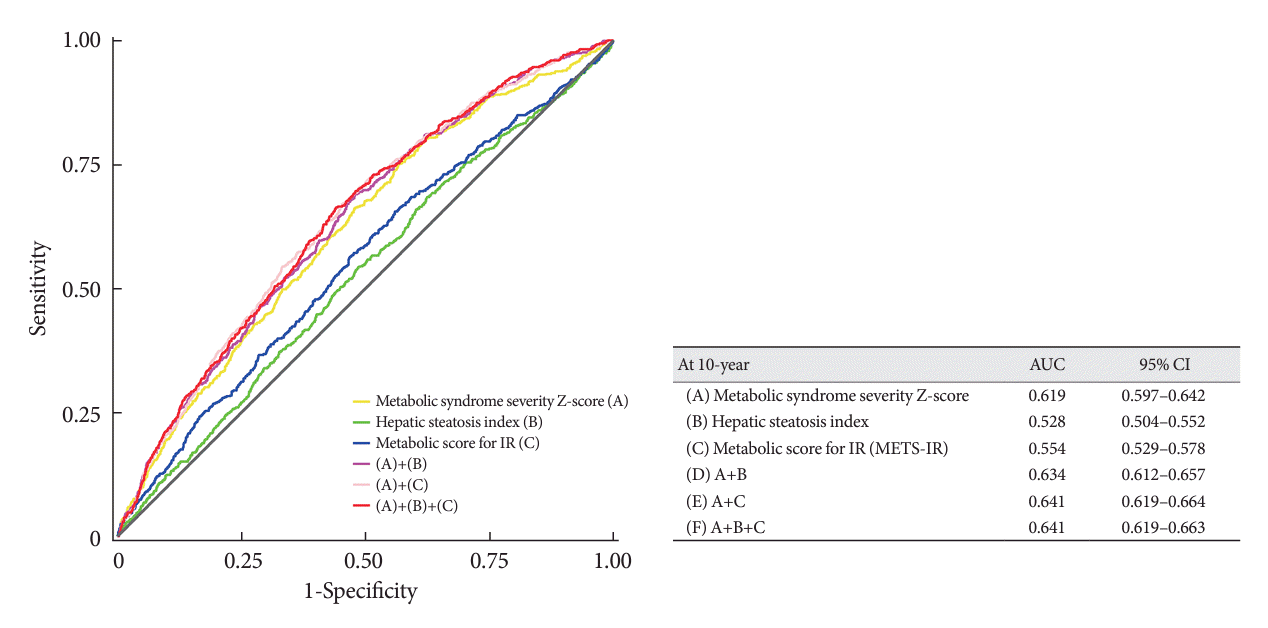
MACCE predictability for each metabolic index
Table 2.
| Variable |
Hazard ratios (95% confidence interval) |
|||
|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | Model 3c | |
| Metabolic syndrome component | ||||
| ≥3 | 1.938 (1.720–2.183) | 1.624 (1.438–1.834) | 1.463 (1.274–1.679) | 1.309 (1.135–1.510) |
| Metabolic syndrome component | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.662 (1.349–2.047) | 1.436 (1.165–1.770) | 1.397 (1.125–1.735) | 1.374 (1.108–1.705) |
| 2 | 2.088 (1.697–2.569) | 1.654 (1.342–2.038) | 1.610 (1.289–2.011) | 1.563 (1.259–1.940) |
| 3 | 2.733 (2.212–3.376) | 2.093 (1.690–2.591) | 1.935 (1.531–2.446) | 1.819 (1.453–2.276) |
| 4 | 3.520 (2.783–4.451) | 2.441 (1.923–3.098) | 2.236 (1.710–2.924) | 1.981 (1.536–2.554) |
| 5 | 4.617 (3.282–6.496) | 3.319 (2.351–4.686) | 3.090 (2.132–4.479) | 2.546 (1.771–3.660) |
| Metabolic syndrome severity Z-score | ||||
| Cut-off value of score | ||||
| >–0.046 | 1.974 (1.748–2.230) | 1.645 (1.455–1.861) | 1.539 (1.330–1.781) | 1.489 (1.306–1.698) |
| Tertiles of score | ||||
| Tertile 1 (–2.612 to –0.473) | Reference | Reference | Reference | Reference |
| Tertile 2 (–0.473 to 0.363) | 1.577 (1.338–1.859) | 1.363 (1.156–1.608) | 1.376 (1.160–1.633) | 1.321 (1.113–1.569) |
| Tertile 3 (0.363 to 9.359) | 2.451 (2.102–2.858) | 1.931 (1.653–2.256) | 2.000 (1.703–2.348) | 1.730 (1.463–2.044) |
| Metabolic syndrome severity score | ||||
| Cut-off value of score | ||||
| >0.997 | 1.990 (1.770–2.237) | 1.816 (1.599–2.063) | 1.695 (1.461–1.967) | 1.513 (1.298–1.764) |
| Tertiles of score | ||||
| Tertile 1 (–2.306 to 0.116) | Reference | Reference | Reference | Reference |
| Tertile 2 (0.116 to 0.922) | 1.234 (1.049–1.451) | 1.261 (1.068–1.487) | 1.343 (1.131–1.593) | 1.298 (1.093–1.541) |
| Tertile 3 (0.922 to 10.309) | 2.166 (1.870–2.510) | 2.008 (1.708–2.359) | 2.083 (1.762–2.463) | 1.794 (1.507–2.135) |
| Hepatic steatosis index | ||||
| Cut-off value of score | ||||
| >31.295 | 1.217 (1.075–1.377) | 1.416 (1.247–1.607) | 1.292 (1.087–1.537) | 1.204 (1.011–1.434) |
| Tertiles of score | ||||
| Tertile 1 (17.185 to 30.778) | Reference | Reference | Reference | Reference |
| Tertile 2 (30.778 to 34.700) | 1.078 (0.929–1.251) | 1.248 (1.074–1.451) | 1.296 (1.109–1.514) | 1.199 (1.025–1.403) |
| Tertile 3 (34.700 to 55.771) | 1.294 (1.121–1.494) | 1.603 (1.383–1.857) | 1.702 (1.461–1.983) | 1.464 (1.250–1.715) |
| Fatty liver index | ||||
| Cut-off value of score | ||||
| >30.421 | 1.647 (1.465–1.852) | 1.531 (1.360–1.723) | 1.454 (1.245–1.698) | 1.421 (1.253–1.611) |
| Tertiles of score | ||||
| Tertile 1 (0.600 to 15.390) | Reference | Reference | Reference | Reference |
| Tertile 2 (15.390 to 40.114) | 1.405 (1.202–1.643) | 1.235 (1.056–1.445) | 1.240 (1.054–1.458) | 1.167 (0.991–1.374) |
| Tertile 3 (40.114 to 99.117) | 1.878 (1.619–2.179) | 1.681 (1.447–1.953) | 1.752 (1.500–2.045) | 1.523 (1.298–1.787) |
| NAFLD liver fat score | ||||
| Cut-off value of score | ||||
| >1.576 | 1.657 (1.472–1.864) | 1.531 (1.360–1.725) | 1.443 (1.240–1.681) | 1.393 (1.227–1.582) |
| Tertiles of score | ||||
| Tertile 1 (–5.032 to –0.806) | Reference | Reference | Reference | Reference |
| Tertile 2 (–0.806 to 3.585) | 1.424 (1.217–1.667) | 1.265 (1.080–1.482) | 1.246 (1.058–1.468) | 1.183 (1.003–1.395) |
| Tertile 3 (3.585 to 38.000) | 1.982 (1.707–2.300) | 1.780 (1.531–2.069) | 1.835 (1.572–2.143) | 1.588 (1.352–1.865) |
| Fibrosis-4 index | ||||
| Cut-off value of score | ||||
| >1.164 | 1.765 (1.571–1.984) | 0.980 (0.860–1.117) | 1.047 (0.915–1.198) | 1.073 (0.938–1.228) |
| Tertiles of score | ||||
| Tertile 1 (0.281 to 0.856) | Reference | Reference | Reference | Reference |
| Tertile 2 (0.856 to 1.193) | 1.257 (1.073–1.472) | 0.850 (0.721–1.002) | 0.869 (0.733–1.030) | 0.872 (0.735–1.033) |
| Tertile 3 (1.193 to 50.836) | 2.022 (1.747–2.341) | 0.888 (0.748–1.053) | 0.968 (0.811–1.154) | 0.996 (0.835–1.187) |
| TyG index | ||||
| Cut-off value of score | ||||
| >8.716 | 1.499 (1.334–1.685) | 1.384 (1.231–1.556) | 1.292 (1.139–1.465) | 1.212 (1.067–1.376) |
| Tertiles of score | ||||
| Tertile 1 (7.148 to 8.370) | Reference | Reference | Reference | Reference |
| Tertile 2 (8.370 to 8.878) | 1.418 (1.215–1.655) | 1.211 (1.037–1.414) | 1.121 (0.954–1.319) | 1.099 (0.935–1.293) |
| Tertile 3 (8.878 to 11.716) | 1.781 (1.535–2.065) | 1.545 (1.330–1.793) | 1.399 (1.192–1.641) | 1.288 (1.095–1.514) |
| HOMA-IR | ||||
| Cut-off value of score | ||||
| >1.566 | 1.370 (1.218–1.540) | 1.462 (1.299–1.645) | 1.377 (1.213–1.563) | 1.306 (1.150–1.484) |
| Tertiles of score | ||||
| Tertile 1 (0.018 to 1.235) | Reference | Reference | Reference | Reference |
| Tertile 2 (1.235 to 1.923) | 0.981 (0.844–1.139) | 1.071 (0.921–1.244) | 1.085 (0.929–1.268) | 1.079 (0.924–1.261) |
| Tertile 3 (1.923 to 59.107) | 1.331 (1.156–1.532) | 1.445 (1.253–1.666) | 1.365 (1.170–1.593) | 1.273 (1.089–1.487) |
| METS-IR | ||||
| Cut-off value of score | ||||
| >35.311 | 1.440 (1.275–1.627) | 1.462 (1.294–1.652) | 1.425 (1.198–1.695) | 1.404 (1.232–1.600) |
| Tertiles of score | ||||
| Tertile 1 (19.605 to 33.507) | Reference | Reference | Reference | Reference |
| Tertile 2 (33.507 to 39.308) | 1.126 (0.967–1.311) | 1.176 (1.009–1.370) | 1.243 (1.060–1.456) | 1.173 (0.999–1.376) |
| Tertile 3 (39.308 to 71.416) | 1.520 (1.317–1.755) | 1.595 (1.381–1.841) | 1.707 (1.470–1.983) | 1.487 (1.274–1.736) |
MACCE, major adverse cardiovascular and cerebrovascular events; NAFLD, nonalcoholic fatty liver disease; TyG, triglyceride-glucose index; HOMA-IR, homeostatic model assessment of insulin resistance; METS-IR, metabolic score for insulin resistance.
Fig. 2.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download