Abstract
Background
We aimed to evaluate the utility of repeat biopsy of thyroid nodules classified as atypia of undetermined significance with architectural atypia (IIIB) on core-needle biopsy (CNB).
Methods
This retrospective study evaluated patients with thyroid nodules categorized as IIIB on CNB between 2013 and 2015. Demographic characteristics, subsequent biopsy results, and ultrasound (US) images were evaluated. The malignancy rates of nodules according to number of CNBs and the number of IIIB diagnoses was compared. Demographic and US features were evaluated to determine factors predictive of malignancy.
Results
Of 1,003 IIIB nodules on CNB, the final diagnosis was determined for 328 (32.7%) nodules, with 121 of them confirmed as malignant, resulting in a malignancy rate of 36.9% (95% confidence interval, 31.7% to 42.1%). Repeat CNB was performed in 248 nodules (24.7%), with 75 (30.2%), 131 (52.8%), 13 (5.2%), 26 (10.5%), one (0.4%), and two (0.8%) reclassified into categories II, IIIB, IIIA, IV, V, and VI, respectively. Malignancy rates were not significantly affected by the number of CNBs (P=0.291) or the number of IIIB diagnoses (P=0.473). None of the nodules confirmed as category II on repeat CNB was malignant. US features significantly associated with malignancy (P<0.003) included solid composition, irregular margins, microcalcifications, and high suspicion on the US risk stratification system.
Although ultrasound (US)-guided fine-needle aspiration (FNA) is generally regarded as the standard diagnostic approach for thyroid nodules [1], the use of core-needle biopsy (CNB) is increasing due to its high diagnostic accuracy, safety, and tolerability [2,3]. CNB has been shown to effectively reduce the rate of nondiagnostic results in thyroid nodules that were previously identified as nondiagnostic results on FNA [4,5]. Additionally, CNB specimens offer a more conclusive diagnosis for various specific diseases, such as malignant lymphoma, medullary thyroid carcinoma, and anaplastic thyroid carcinoma [6,7]. Moreover, CNB is particularly effective in cases of nodules with macrocalcification or degeneration [8-10]. Therefore, CNB has been increasingly utilized as a first-line diagnostic tool for initially detected thyroid nodules [11]. However, recent meta-analysis reported that CNB had higher proportion of indeterminate follicular lesions than FNA, with 72% of nodules diagnosed as indeterminate follicular lesions on CNB being due to architectural atypia [12].
Current guidelines and the results of recent studies recommend various management options for nodules diagnosed on FNA as atypia of undetermined significance (AUS)/follicular lesions of undetermined significance (FLUS), including repeat biopsy, molecular testing, follow-up, or diagnostic surgery [1,13,14]. To date, however, few studies have assessed appropriate management strategies and the role of repeat biopsy for nodules diagnosed on CNB as indeterminate follicular lesions with architectural atypia. The present study therefore evaluated the role of repeat biopsy in the diagnosis of these nodules, as well as determining the US features predictive of malignancy.
This retrospective study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB approval number: 2018-1307). Informed consent had been obtained from all patients prior to thyroid CNB.
The present study enrolled consecutive patients who underwent US-guided CNB of thyroid nodules at our institution between January 2013 and December 2015. Patients were included if they were aged ≥18 years and if the histopathologic results of CNB showed indeterminate follicular lesions with architectural atypia. Clinical features, US findings, and histopathologic records of each patient were reviewed.
US examination was performed using an iU22 or HDI-5000 unit (Philips Healthcare, Bothell, WA, USA) or an EUB-7500 unit (Hitachi Medical Systems, Tokyo, Japan), each equipped with a linear high-frequency probe with ranging from 5 to 14 MHz. All US scans and US-guided CNBs were carried out by radiologists, supervised by senior staff radiologists, with more than 14 years of clinical expertise in evaluating thyroid US images. The decision to perform CNB or FNA was based on the preference of the operator. Detailed information on technical methods on US-guided CNB biopsy is provided in Supplemental Methods. If patients reported pain or swelling in the neck, a follow-up US examination was conducted to assess possible complication.
Two radiologists (S.R.C. and H.H.M. with 8 and 2 years of experience in thyroid US imaging, respectively), who were blinded to the clinicopathologic data, evaluated nodule features retrospectively. Consensus results were obtained by resolving any discrepancies in individual interpretations. Among the nodules features evaluated were internal composition (solid or partially solid), echogenicity (hyperechoic, isoechoic, hypoechoic, or markedly hypoechoic), margins (well-defined, ill-defined, or irregular), calcification (no calcifications, rim calcifications, macrocalcification, or microcalcifications), orientation (parallel or nonparallel), presence of halo, presence of central vascularity, and Korean Thyroid Imaging Reporting and Data System (K-TIRADS) category according to the guidelines of the Korean Society of Thyroid Radiology [15].
CNB and surgical specimens were examined by a staff pathologist with 18 years of expertise in thyroid cytopathology. The CNB results were classified into six categories based on the guidelines of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group [16]: nondiagnostic or unsatisfactory (category I), benign lesion (category II), indeterminate follicular lesion (category III), follicular neoplasm/suspicious for follicular neoplasm (category IV), suspicious for malignancy (category V), and malignant (category VI). Within the category of indeterminate follicular lesions (category III), further subcategories were identified as indeterminate follicular lesions with nuclear atypia (category IIIA), indeterminate follicular lesion with architectural atypia (category IIIB), and other indeterminate lesions (category IIIC) (Fig. 1).
Following a histopathologic review, immunohistochemical staining for Hector Battifora mesothelial-1 (HBME-1) and galectin-3, as well as DNA mutational analysis of the BRAFV600E and RAS genes, were performed. The decisions to perform these additional tests in the residual biopsy specimen were at the discretion of both the physician without specified protocol. Detailed information on the technical methods employed for immunohistochemistry and molecular testing is provided in the Supplemental Methods. The surgery was decided upon through comprehensive consideration of various factors, including US findings, size, immunohistochemical staining, BRAF and RAS mutation results, in consultation between the patient and physician.
Histopathologic results from surgery and repeat CNB, as well as clinical follow-up with US, were used as the standard reference. Final diagnosis of malignancy was based on histopathologic findings from surgical resection or biopsy. A final diagnosis of benign nodule was made when one of the following conditions was fulfilled: benign results on surgical diagnosis or benign results on subsequent CNB and decreased or stable size at follow-up US more than 24 months later.
Malignancy rates relative to the number of CNBs and the number of nodules classified as IIIB were compared using chi-square tests. Factors predictive of malignancy in nodules with IIIB results were identified by comparing the clinical and US features of benign and malignant nodules. Categorical variables were assessed using chi-square and Fisher’s exact tests, while continuous variables were analyzed using two-sample t tests. Major and minor complications were identified according to the Society of Interventional Radiology definitions [17,18]. All statistical analyses were performed using SPSS version 21.0 for Windows (IBM Corp., Armonk, NY, USA) and MedCalc version 19.1 (MedCalc Software, Mariakerke, Belgium) software, with P values <0.05 defined as statistically significant.
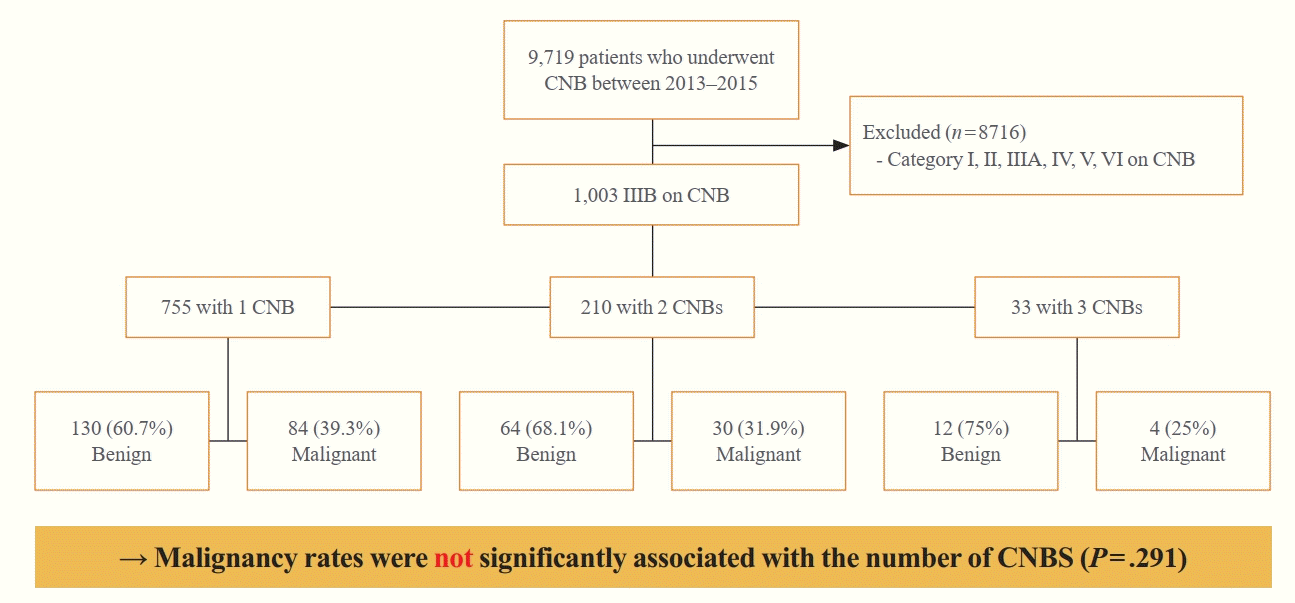
Of the 9,719 thyroid nodules evaluated by CNB between January 2013 and December 2015, 1,003 (10.3%) nodules in 975 patients, consisting of 787 women and 188 men, of mean age 53.9 years, exhibited IIIB at least once. Some nodules underwent a single biopsy, whereas others were biopsied repeatedly to refine the diagnoses. Of these 1,003 nodules, the final diagnosis was determined for 328 (32.7%) nodules. Among these 328 nodules, 289 nodules were determined through surgery, nine nodules through subsequent CNB, and 30 nodules through follow-up US. Table 1 shows the baseline characteristics and US features of these patients.
Baseline Demographic and Clinical Characteristics of the Patients Included in This Study
Of the 1,003 nodules with IIIB on CNB, 121 were malignant, 207 were benign, and 675 were not confirmed, yielding a malignancy rate of 36.9% (95% confidence interval [CI], 31.7% to 42.1%). Fig. 2 shows the malignancy rate according to the number of CNBs. Of the 1,003 nodules diagnosed with IIIB, 755 (75.3%), 210 (20.9%), and 33 (3.3%) underwent one, two and three CNBs, respectively. Of the 755 nodules that underwent CNB once, 84 were malignant, 130 were benign, and 541 were not confirmed, yielding a malignancy rate of 39.3% (95% CI, 32.7% to 45.8%). Of the 210 nodules that underwent CNB twice, 30 were malignant, 64 were benign, and 116 were not confirmed, yielding a malignancy rate of 31.9% (95% CI, 22.5% to 41.3%). Of the 33 nodules that underwent CNB three times, four were malignant, 12 were benign, and 17 were not confirmed, yielding a malignancy rate of 25%. The malignancy rates for nodules that underwent one, two, and three CNBs were 39.3%, 31.9%, and 25%, respectively. Malignancy rates were not significantly associated with the number of CNBs (P=0.291).
Out of the 1,003 thyroid nodules that were classified as IIIB on initial CNB, 214 (21.3%) underwent direct surgery and 248 (24.7%) underwent repeat CNB. Direct surgery was more frequently chosen for nodules larger than 2 cm (70.1% vs. 44.0%, P<0.0001) and for nodules with central vascularity (79.9% vs. 69.8%, P<0.0001) (Supplemental Table S1).
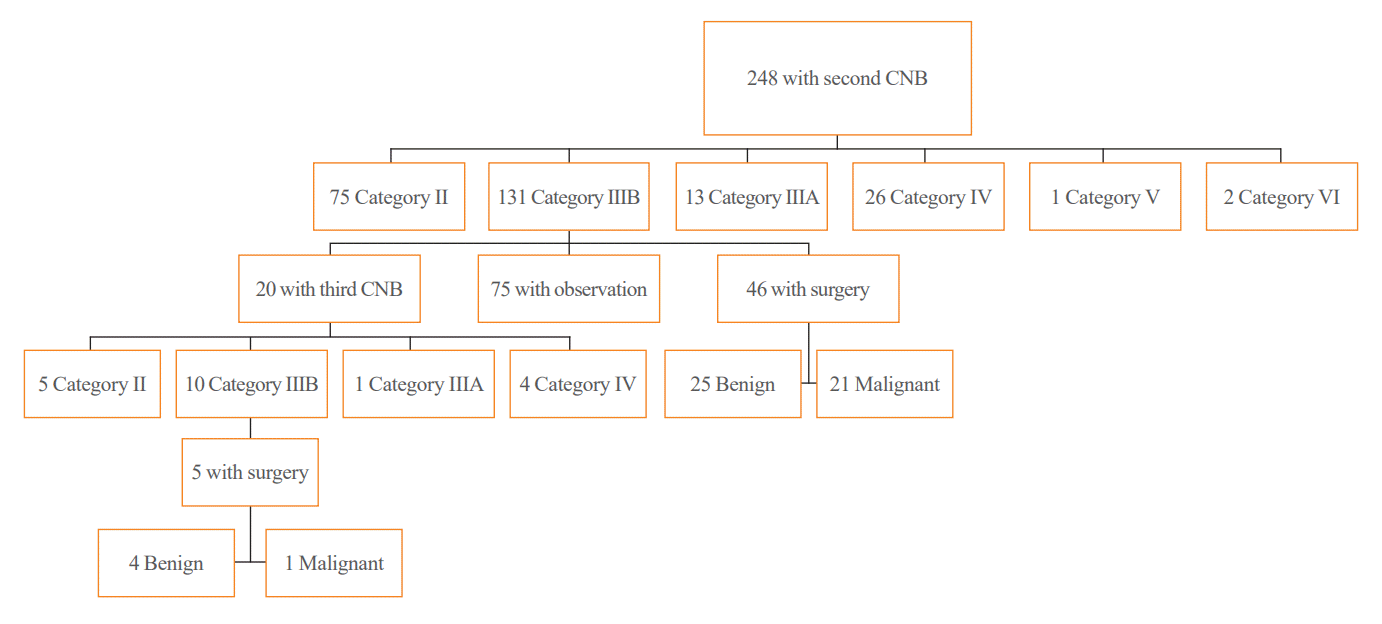
Of the 248 nodules that underwent a second CNB, 75 (30.2%), 131 (52.8%), 13 (5.2%), 26 (10.5%), one (0.4%), and two (0.8%) were classified as category II, IIIB, IIIA, IV, V, and VI, respectively (Fig. 3). Of the 131 nodules with two consecutive IIIB diagnoses, 46 (35.1%) underwent direct surgery and 20 (15.2%) underwent a third CNB. There were no significant differences in clinicoradiologic features between the group that underwent direct surgery and the group that underwent third CNB (Supplemental Table S2). Of the 131 nodules with two consecutive IIIB diagnoses, 21 were malignant, 25 were benign, and 85 were not confirmed, yielding a malignancy rate of 45.7% (95% CI, 31.3% to 60.0%).
Of the 20 nodules that underwent a third CNB, five (25%), 10 (50%), one (5%), and four (20%) were classified as category II, IIIB, IIIA, and IV, respectively. Of those 10 nodules with three consecutive IIIB diagnoses, 1 were malignant, 4 were benign, and 5 were not confirmed, yielding a malignancy rate of 20%. Overall, the malignancy rates for nodules diagnosed with 1, 2, and 3 consecutive IIIB were 39.3%, 45.7%, and 20%, respectively. There was no statistically significant difference in the malignancy rates according to number of IIIB diagnoses (P=0.473). The diagnostic performance of the second and third CNBs was provided in Supplemental Tables S3, S4.
Table 2 presents the malignancy rates for each category of nodules based on the results of repeat CNB. Of the 75 nodules classified as II, six exhibited benign results on surgery, eight showed benign results on subsequent CNB, and 30 demonstrated decreased or stable size during follow-up US, confirming their benign nature. Consequently, out of the 75 nodules, 44 were classified as benign, while none were determined to be malignant, resulting in a malignancy rate of 0%. Of the 13 nodules classified as IIIA, two were malignant, two were benign, and nine were not confirmed, yielding a malignancy rate of 45.7%. Of the 26 nodules classified as IV, 11 were malignant, six were benign, and nine were not confirmed, yielding a malignancy rate of 64.7%. The one nodule classified as category V underwent surgery and was malignant. Two nodules classified as category VI underwent surgery and was malignant. Thus, the malignancy rates were 0%, 45.7%, 50%, 64.7%, 100%, and 100% for nodules of categories II, IIIB, IIIA, VI, V, and VI, respectively.
Results of Second Core-Needle Biopsy after Initial IIIB Diagnosis
The final histopathologic outcomes for IIIB nodules are shown in Table 3. Of the 289 nodules that underwent surgery, 169 (58.5%) were benign and 120 (41.5%) were malignant. Of the 169 benign nodules, 88 (52.1%) were thyroid follicular nodular disease, 78 (46.1%) were follicular thyroid adenomas, and three (1.8%) were parathyroid adenomas. Of the 120 malignant nodules, 66 (55%) were follicular variant papillary thyroid carcinomas (PTCs), 45 (37.5%) were minimally invasive follicular thyroid carcinomas, seven (5.8%) were classic PTCs, and two (1.7%) were oncocytic variant PTCs. Out of the 120 malignant tumors, 111 patients were classified as stage I, while nine patients were classified as stage II.
Final Histopathologic Outcomes for IIIB Nodules Triaged to Surgery
Table 4 shows a comparison of US features and immunohistochemistry of IIIB nodules categorized on final diagnosis as benign or malignant. Malignancy was significantly associated with nodules having solid composition (80.8% vs. 47.9%, P=0.030), irregular margins (11.7% vs. 0%, P<0.001), microcalcifications (10.8% vs. 3.6%, P=0.014), and K-TIRADS 5 (11.7% vs. 3.6%, P=0.007). The proportion of K-TIRADS 3 was significantly higher in benign than in malignant nodules (69.2% vs. 53.3%, P=0.005). The malignancy rates were 55.4% (92/166) for solid nodules, 100% (15/15) for nodules with irregular margins, 70% (14/20) for nodules with microcalcifications, and 71.4% (15/21) for nodules classified as K-TIRAD 5.
Ultrasonographic Features and Immunohistochemistry/Molecular Test of IIIB Nodules Diagnosed as Benign or Malignant
There was no statistically significant difference observed between benign and malignant nodules in terms of immunohistochemistry/molecular test of HBME-1, galectin-3, BRAFV600E, and RAS.
None of the included patients experienced any major complications related to the intervention or hospitalization. Only two patients developed a hematoma following the procedure, resulting in a complication rate of 0.2% (two out of 1,003). The hematoma was successfully resolved with 1 hour of compression.
The present study revealed that nodules categorized as IIIB on CNB have a substantial malignancy rate of 36.9% (95% CI, 31.7% to 42.1%). Malignancy rates were not significantly associated with the numbers of CNBs or IIIB diagnoses, with 52.8% of the nodules that underwent repeat CNB yielding consistent IIIB results. However, none of the nodules confirmed as category II on repeat CNB were malignant. Notably, several US features, including solid composition, irregular margins, microcalcifications, and K-TIRADS 5 classification, were associated with malignancy.
The use of CNB is increasing due to its advantages of CNB over FNA, including enhanced diagnostic accuracy and lower rates of nondiagnostic results. There are standardized diagnostic categories for thyroid CNB, which are similar to the Bethesda categories used for FNA [16]. Repeat biopsy is generally recommended for nodules classified on FNA as Bethesda category III (AUS/FLUS). For example, the 2015 American Thyroid Association guidelines have a weak recommendation for repeat FNA [1] and the American Association of Clinical Endocrinologists/Associazione Medici Endocrinologi/European Thyroid Association guidelines recommend conservative management or repeat FNA considering the personal history, lesion size, and US and elastography features [19]. However, the role of repeated biopsy for indeterminate lesions on CNB has not been investigated. Because the incidence of architectural atypia in indeterminate lesions is higher on CNB than on FNA, and malignancy rate differs significantly in lesions with architectural and nuclear atypia [20], the present study investigated the role of repeat biopsy for nodules classified as IIIB on CNB.
The present study found that the malignancy rate on CNB was 36.9% (95% CI, 31.7% to 42.1%), rates comparable to or higher than those reported for nodules classified as Bethesda category III on FNA [21,22]. The rate of repeat IIIB diagnosis on repeat CNB in the present study was 52.8%, higher than the reported rate of repeat AUS/FLUS diagnosis on repeat FNA, which was 26% (95% CI, 20% to 32%) [23]. Furthermore, there was no significant difference in malignancy rate observed when surgery was performed after subsequent biopsies and repeated IIIB results. These findings suggest that repeat biopsy for nodules initially diagnosed as IIIB on CNB may be ineffective.
Following a second CNB, 30.2% IIIB of nodules were classified as benign lesions, and none of them were found to be malignant, resulting in a malignancy rate of 0%. Despite only six patients undergoing surgery, eight nodules were confirmed as benign on subsequent CNB, and 30 nodules demonstrated decreased or stable size over a follow-up period of more than 2 years (mean±standard deviation duration of follow-up, 5.8±2.7 years). According to a recent meta-analysis, 48% of nodules initially classified as AUS/FLUS are reclassified as benign on repeat FNA, with reported malignancy rates for these resected nodules at 4% (95% CI, 1% to 7%) [23]. Although our study demonstrates a lower rate of reclassification to benign with repeat CNB compared to FNA, it is noteworthy that none of the nodules reclassified as benign on repeat CNB were diagnosed as malignancy, indicating a false negative rate of 0%. Therefore, approximately 30% of patients who receive a benign result on repeat biopsy can potentially avoid unnecessary surgery based on these findings.
The high malignancy rate of IIIB nodules and the limitations of repeat biopsy suggest that repeat biopsy be performed in selected, rather than in all patients. The present study confirmed that nodules with features such as solid composition, irregular margins, microcalcifications, and K-TIRADS 5 on US are associated with malignancy. Similarly, Kaya et al. [24] and Evranos Ogmen et al. [25] reported that suspicious US features, such as irregular margins, microcalcifications, and hypoechogenicity, were associated with malignancy in patients with FNA results of AUS/FLUS. Kuru et al. [26] found that microcalcifications, solid composition, hypoechogenicity, irregular margins, and increased vascularity of nodules have been associated with malignancy, suggesting the need for surgical treatment. According to our study, the malignancy rates were 55.4% (92/166) for solid nodules, 100% (15/15) for nodules with irregular margins, 70% (14/20) for nodules with microcalcifications, and 71.4% (15/21) for nodules categorized as K-TIRAD 5. Considering that nodules with irregular margins, microcalcifications, and K-TIRAD 5 result have a malignancy rate of 70% or higher, it is advisable to consider diagnostic surgery instead of repeating the biopsy.
The malignancy rate of nodules classified as IIIB in our study was found to be similarly high as previous reported malignancy rate of nodules in category IV; however, there are considerations to take into account in this regard. One factor to consider is the potential for overestimation of malignancy risk, given that only 28.8% of the lesions undergo surgery. Additionally, it should be noted that diagnosing follicular pattern lesions in CNB specimens can be particularly challenging for pathologists, and the diagnostic rates within these CNB categories can fluctuate depending on the diagnostic thresholds used by pathologists. It has been reported that the diagnostic rates for categories II, III, and IV can vary significantly among different institutions [20,27,28]. For instance, if a microfollicular proliferative lesion is present and distinctly separated from the surrounding normal parenchyma by a fibrous capsule, it would be categorized as IV. On the other hand, if a CNB specimen exhibits primarily a microfollicular or trabecular growth pattern but lacks a discernible fibrous capsule or adjacent nonlesional tissue, it would be diagnosed as IIIB [29]. Such diagnoses are influenced by whether or not a capsule is included in the specimen. For these reasons, recently, there has been study proposing the subdivision of follicular-patterned lesions in CNB categories III and IV based on nuclear atypia and RAS mutations [30]. In our study, while immunohistochemical or molecular testing, including RAS, did not show a significant difference in distinguishing between benign and malignant cases, this analysis was constrained by the limited number of patients who underwent these tests. Therefore, further research on the subcategorization of follicular pattern lesions is deemed necessary in the future.
This study had several limitations. First, only 28.8% of nodules diagnosed with IIIB underwent surgical resection and therefore the true malignancy rate may differ from the estimated rate seen in our study. However, we made efforts to determine the final diagnosis of as many nodules as possible and accurately estimate the true malignancy rate by investigating subsequent CNB results and examining the changes in nodule size during the patients’ 2-year follow-up. Second, because this was a retrospective study, decisions on whether to perform surgery or repeat biopsy were often based on the preferences of the clinician and the patient, which may have introduced selection bias. Third, the absence of internationally standardized diagnostic pathology criteria for CNB diagnosis in thyroid nodules, as well as the potential variation in CNB category rates among different pathologists, could impede the generalizability of the study findings. In addition, this study included patients at a single-center study and multigene panel were not performed to assess the malignancy rate.
In conclusion, repeat biopsy for nodules with IIIB on CNB did not enhance the identification of malignancy but could potentially reduce unnecessary surgery. Repeat biopsy may benefit selected patients, with US features guiding the choice between repeat biopsy and diagnostic surgery.
Supplementary Material
Supplemental Table S1.
Baseline Demographic and Clinical Characteristics of the Patients Who Underwent Direct Surgery and Those Who Did Not
Supplemental Table S2.
Baseline Demographic and Clinical Characteristics of the Patients with Two Consecutive IIIB Diagnoses
Supplemental Table S3.
Diagnostic Performance of the Second Core-Needle Biopsy for the Diagnosis of Malignancy
Supplemental Table S4.
Diagnostic Performance of the Third Core-Needle Biopsy for the Diagnosis of Malignancy
REFERENCES
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

2. Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology. 2003; 226:827–32.

3. Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. 2014; 24:2819–26.

4. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2012; 22:468–75.

5. Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–72.

6. Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016; 26:1031–6.

7. Ha EJ, Baek JH, Na DG, Kim JH, Kim JK, Min HS, et al. The role of core needle biopsy and its impact on surgical management in patients with medullary thyroid cancer: clinical experience at 3 medical institutions. AJNR Am J Neuroradiol. 2015; 36:1512–7.

8. Ha EJ, Baek JH, Lee JH, Kim JK, Kim JK, Lim HK, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–9.

9. Ha EJ, Baek JH, Lee JH, Lee HY, Song DE, Kim JK, et al. A focal marked hypoechogenicity within an isoechoic thyroid nodule: is it a focal malignancy or not? Acta Radiol. 2015; 56:814–9.

10. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–8.
11. Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403.

12. Ahn SH. Usage and diagnostic yield of fine-needle aspiration cytology and core needle biopsy in thyroid nodules: a systematic review and meta-analysis of literature published by Korean authors. Clin Exp Otorhinolaryngol. 2021; 14:116–30.

13. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.

14. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest. 2010; 33:287–91.

15. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021; 22:2094–123.
16. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–99.
17. Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S243–6.

18. Lewis CA, Allen TE, Burke DR, Cardella JF, Citron SJ, Cole PE, et al. Quality improvement guidelines for central venous access: the standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1997; 8:475–9.
19. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–39.
20. Chung SR, Baek JH, Lee JH, Lee YM, Sung TY, Chung KW, et al. Risk of malignancy according to the sub-classification of atypia of undetermined significance and suspicious follicular neoplasm categories in thyroid core needle biopsies. Endocr Pathol. 2019; 30:146–54.

21. Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid. 2014; 24:832–9.

22. Straccia P, Rossi ED, Bizzarro T, Brunelli C, Cianfrini F, Damiani D, et al. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015; 123:713–22.

23. Bayona A, Benavent P, Muriel A, Abuchaibe C, Sharpe SC, Tarasova V, et al. Outcomes of repeat fineneedle aspiration biopsy for AUS/FLUS thyroid nodules. Eur J Endocrinol. 2021; 185:497–506.

24. Kaya C, Bozkurt E, Turkyilmaz Mut D, Mihmanli M, Uludag M. Which factors are associated with malignancy in thyroid nodules classified as Bethesda category 3 (AUS/FLUS) and how do they influence the patient’s management? Acta Endocrinol (Buchar). 2019; 15:491–6.

25. Evranos Ogmen B, Aydin C, Kilinc I, Aksoy Altinboga A, Ersoy R, Cakir B. Can repeat biopsies change the prognoses of AUS/FLUS nodule? Eur Thyroid J. 2020; 9:92–8.

26. Kuru B, Atmaca A, Tarim IA, Kefeli M, Topgul K, Yoruker S, et al. Risk factors associated with malignancy and with triage to surgery in thyroid nodules classified as Bethesda category III (AUS/FLUS). Eur J Surg Oncol. 2016; 42:87–93.

27. Na HY, Woo JW, Moon JH, Choi JY, Jeong WJ, Kim YK, et al. Preoperative diagnostic categories of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in thyroid core needle biopsy and its impact on risk of malignancy. Endocr Pathol. 2019; 30:329–39.

28. Kim K, Bae JS, Kim JS, Jung SL, Jung CK. Diagnostic performance of thyroid core needle biopsy using the revised reporting system: comparison with fine needle aspiration cytology. Endocrinol Metab (Seoul). 2022; 37:159–69.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download