Introduction
Materials and Methods
1. Study patients
2. Definition of cancer stage
3. Treatment modalities
4. Statistical analyses
Results
1. Baseline characteristics
Fig. 1.
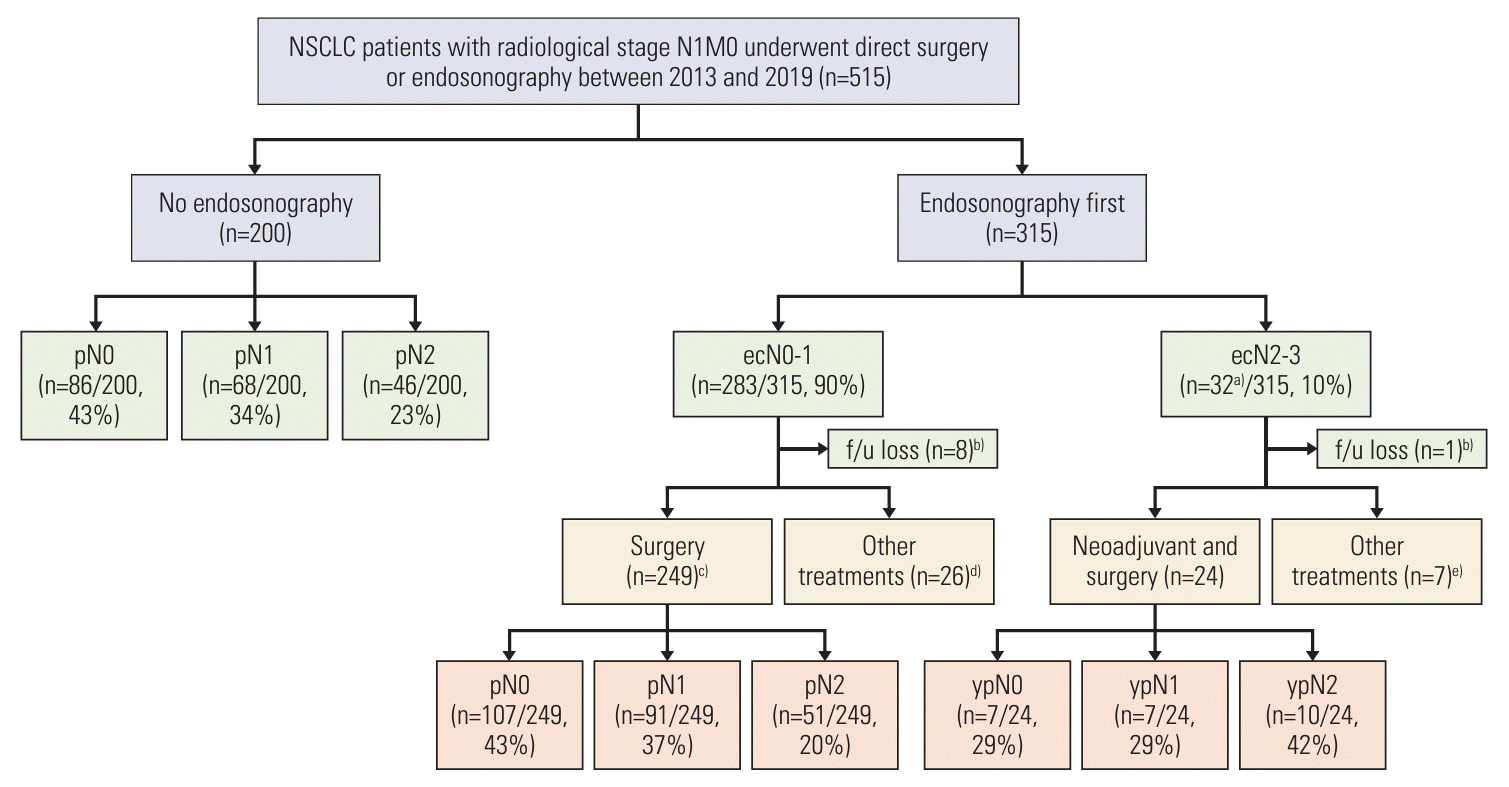
Table 1.
Values are presented as mean±standard deviation or number (%). Matched by age, sex, BMI, smoking status, underlying pulmonary disease, underlying extra-pulmonary comorbidities, clinical T category, and histologic type. BMI, body mass index; NSCLC, non–small cell lung cancer; SD, standardized difference.
2. Detailed treatment profiles of patients undergoing surgery and MLND
Table 2.
| Variable | Total (n=473) | No endosonography (n=200) | Endosonography first (n=273a)) | p-value |
|---|---|---|---|---|
| Duration from visit to surgery (day) | 28 (20-39) | 23 (16-32) | 32 (22-48) | < 0.001 |
| Neoadjuvant treatment | ||||
| No | 447 (94.5) | 200 (100) | 247 (90.5) | < 0.001 |
| CCRT | 26 (5.5) | 0 | 26b) (9.5) | |
| Surgical approach | ||||
| VATS | 160 (33.8) | 79 (39.5) | 81 (29.7) | 0.026 |
| Thoracotomy | 313 (66.2) | 121 (60.5) | 192 (70.3) | |
| Types of surgical resection | ||||
| Sublobar resection | 17 (3.6) | 10 (5.0) | 7 (2.6) | 0.562 |
| Lobectomy | 376 (79.5) | 157 (78.5) | 219 (80.2) | |
| Bilobectomy | 45 (9.5) | 18 (9.0) | 27 (9.9) | |
| Pneumonectomy | 35 (7.4) | 15 (7.5) | 20 (7.3) | |
| Pathologic stage | ||||
| ypCR | 3 (0.6) | 0 | 3 (1.1) | 0.572 |
| IA | 58 (12.3) | 24 (12.0) | 34 (12.5) | |
| IB | 49 (10.4) | 22 (11.0) | 27 (9.9) | |
| IIA | 34 (7.2) | 17 (8.5) | 17 (6.2) | |
| IIB | 146 (30.9) | 58 (29.0) | 88 (32.2) | |
| IIIA | 150 (31.7) | 62 (31.0) | 88 (32.2) | |
| IIIB | 33 (7.0) | 17 (8.5) | 16 (5.9) | |
| Post-operative complicationsc) | 109 (23.0) | 47 (23.5) | 62 (22.7) | 0.840 |
| Pulmonary | 82 (17.3) | 38 (19.0) | 44 (16.1) | 0.413 |
| Cardiovascular | 40 (8.5) | 13 (6.5) | 27 (9.9) | 0.191 |
| Neurologic | 2 (0.4) | 0 | 2 (0.7) | 0.511 |
| Bleeding | 1 (0.2) | 0 | 1 (0.4) | > 0.999 |
| Adjuvant treatment | ||||
| No | 194 (41.0) | 100 (50.0) | 95 (34.8) | 0.001 |
| Yes | 279 (59.0) | 100 (50.0) | 178 (65.2) | |
| CCRT | 59 (12.5) | 24 (12.0) | 35 (12.8) | |
| Chemotherapy | 207 (43.8) | 72 (36.0) | 134 (49.1) | |
| Radiotherapy | 13 (2.7) | 4 (2.0) | 9 (3.3) |
Values are presented as number (%) or median (interquartile range). CCRT, concurrent chemoradiation therapy; MLND, mediastinal lymph nodes dissection; NSCLC, non–small cell lung cancer; VATS, video-assisted thoracoscopic surgery.
3. Effect of nodal staging with endosonography on OS
Fig. 2.
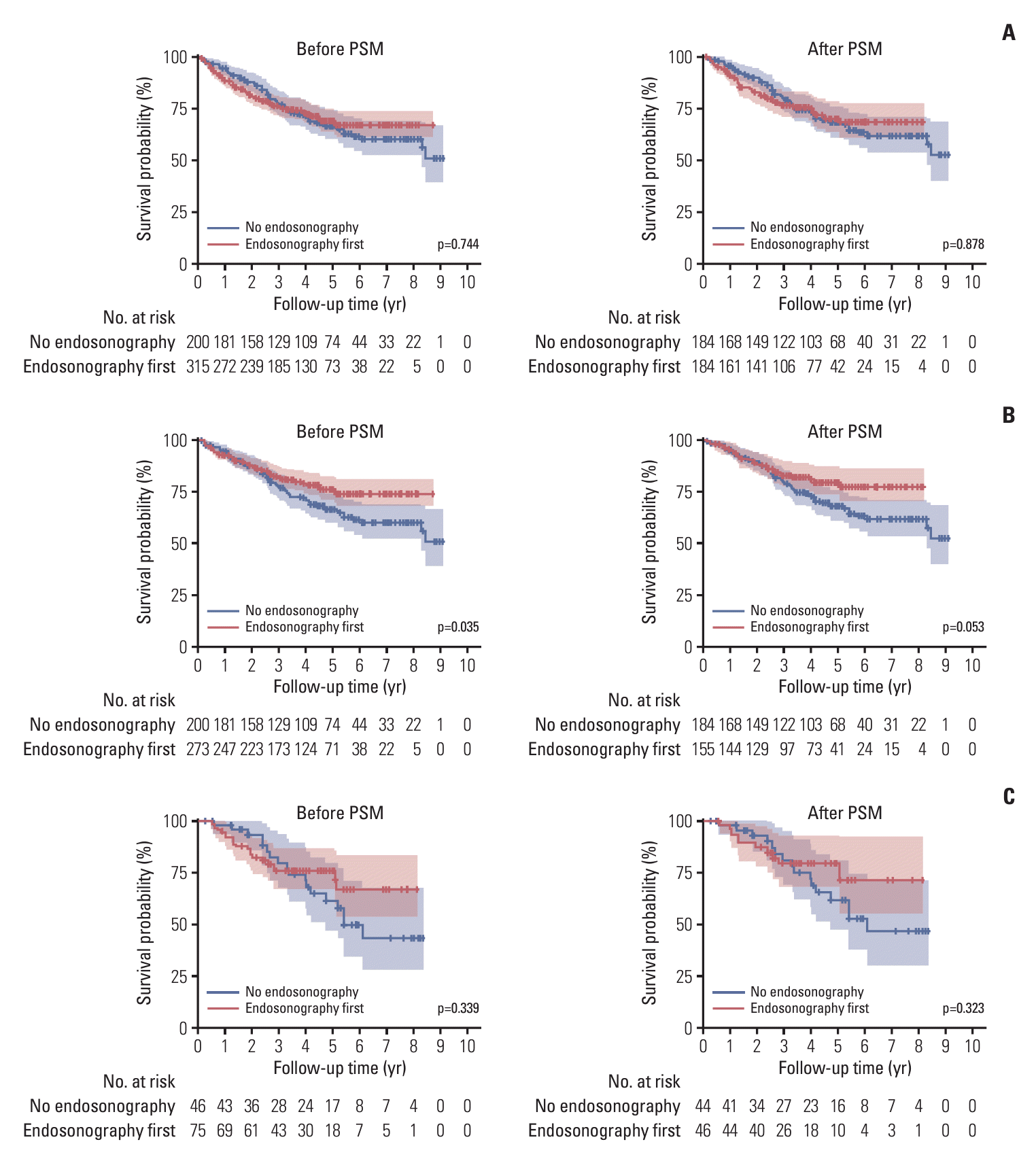
Table 3.
| Subject | Model |
Overall survival |
||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| All patients | ||||
| No endosonography vs. endosonography first | Before PSM (n=200 vs. 315) | |||
| Unadjusted | 0.95 | 0.68-1.31 | 0.743 | |
| Adjusted (model 1)a) | 0.98 | 0.69-1.38 | 0.886 | |
| Adjusted (model 2)b) | 0.97 | 0.69-1.38 | 0.879 | |
| After PSM (n=184 vs. 184) | 0.97 | 0.66-1.43 | 0.880 | |
| Patients who underwent surgery and MLND | ||||
| No endosonography vs. subgroup who underwent surgery in endosonography first group | Before PSM (n=200 vs. 273) | |||
| Unadjusted | 0.68 | 0.47-0.98 | 0.036 | |
| Adjusted (model 1)a) | 0.69 | 0.47-1.02 | 0.061 | |
| Adjusted (model 2)b) | 0.67 | 0.45-0.99 | 0.049 | |
| Adjusted (model 3)c) | 0.66 | 0.44-0.99 | 0.044 | |
| After PSM (n=184 vs. 155) | 0.65 | 0.41-1.01 | 0.054 | |
| Patients with MLN metastases | ||||
| Subgroup with pN2 in the no endosonography group vs. subgroup with eN2-3 or pN2 in endosonography first group | Before PSM (n=46 vs. 75) | |||
| Unadjusted | 0.73 | 0.38-1.40 | 0.341 | |
| Adjusted (model 1)a) | 0.65 | 0.32-1.36 | 0.255 | |
| Adjusted (model 2)b) | 0.62 | 0.29-1.33 | 0.222 | |
| After PSM (n=44 vs. 46) | 0.67 | 0.30-1.49 | 0.326 | |
Matching with age, sex, BMI, smoking status, underlying pulmonary disease, underlying extra-pulmonary comorbidities, clinical T category, and histologic type. BMI, body mass index; CI, confidence interval; eN, endosonographic nodal stage; HR, hazard ratio; MLN, mediastinal lymph nodes; MLND, mediastinal lymph nodes dissection; pN, pathologic nodal stage; PSM, propensity score matching.
a) Adjusted by age, sex, BMI, smoking status, underlying pulmonary disease, underlying extra-pulmonary comorbidities, clinical T category, and histologic type,




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download