1. de Waard AM, Hollander M, Korevaar JC, Nielen MM, Carlsson AC, Lionis C, Seifert B, Thilsing T, de Wit NJ, Schellevis FG, et al. Selective prevention of cardiometabolic diseases: activities and attitudes of general practitioners across Europe. Eur J Public Health. 2019; 29:88–93. PMID:
30016426.
2. Han Y, Hu Y, Yu C, Guo Y, Pei P, Yang L, Chen Y, Du H, Sun D, Pang Y, et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021; 42:3374–3384. PMID:
34333624.
3. Sakakibara BM, Obembe AO, Eng JJ. The prevalence of cardiometabolic multimorbidity and its association with physical activity, diet, and stress in Canada: evidence from a population-based cross-sectional study. BMC Public Health. 2019; 19:1361. PMID:
31651286.
4. Weigand-Heller AJ, Kris-Etherton PM, Beelman RB. The bioavailability of ergothioneine from mushrooms (
Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev Med. 2012; 54(Suppl):S75–S78. PMID:
22230474.
5. Gunawardena D, Bennett L, Shanmugam K, King K, Williams R, Zabaras D, Head R, Ooi L, Gyengesi E, Münch G. Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-γ activated murine macrophages. Food Chem. 2014; 148:92–96. PMID:
24262531.
6. Guggenheim AG, Wright KM, Zwickey HL. Immune modulation from five major mushrooms: application to integrative oncology. Integr Med (Encinitas). 2014; 13:32–44.
7. Yoon KN, Lee JS, Kim HY, Lee KR, Shin PG, Cheong JC, Yoo YB, Alam N, Ha TM, Lee TS. Appraisal of antihyperlipidemic activities of
Lentinus lepideus in hypercholesterolemic rats. Mycobiology. 2011; 39:283–289. PMID:
22783117.
8. Kirbağ S, Akyüz M. Nutritive value of edible wild and cultured mushrooms. Turk J Biol. 2010; 34:97–102.
9. Sahoo S, Gayakwad T, Shahi S. Medicinal value of edible mushrooms: a review. Int J Health Sci (Qassim). 2022; 6:8760–8767.
10. Muszyńska B, Grzywacz-Kisielewska A, Kała K, Gdula-Argasińska J. Anti-inflammatory properties of edible mushrooms: a review. Food Chem. 2018; 243:373–381. PMID:
29146352.
11. Yadav D, Negi PS. Bioactive components of mushrooms: Processing effects and health benefits. Food Res Int. 2021; 148:110599. PMID:
34507744.
12. Krittanawong C, Isath A, Hahn J, Wang Z, Fogg SE, Bandyopadhyay D, Jneid H, Virani SS, Tang WH. Mushroom consumption and cardiovascular health: a systematic review. Am J Med. 2021; 134:637–642.e2. PMID:
33309597.
13. Uffelman CN, Chan NI, Davis EM, Wang Y, McGowan BS, Campbell WW. An assessment of mushroom consumption on cardiometabolic disease risk factors and morbidities in humans: a systematic review. Nutrients. 2023; 15:1079. PMID:
36904079.
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6:e1000100. PMID:
19621070.
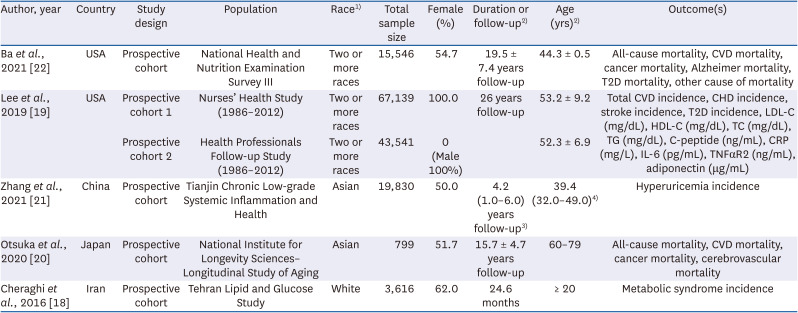
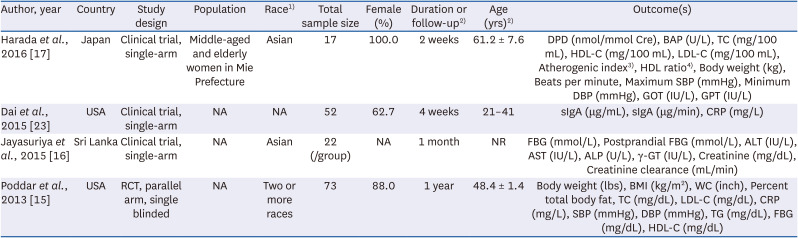
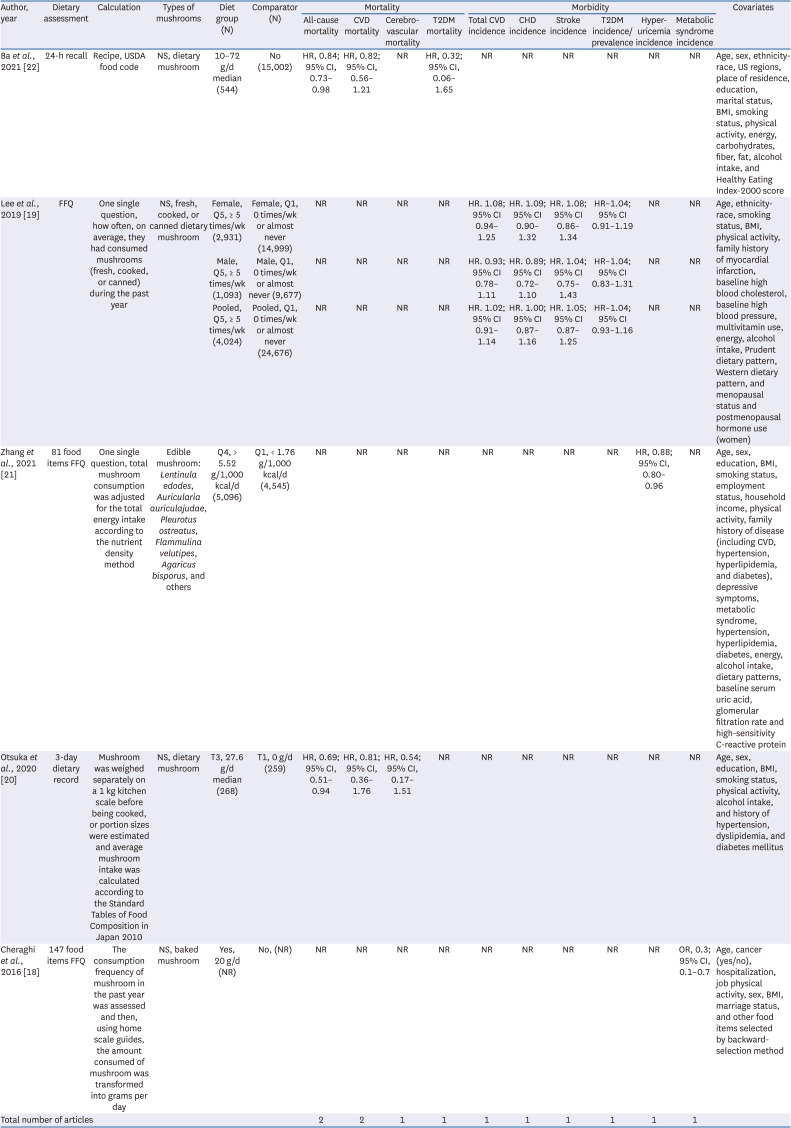
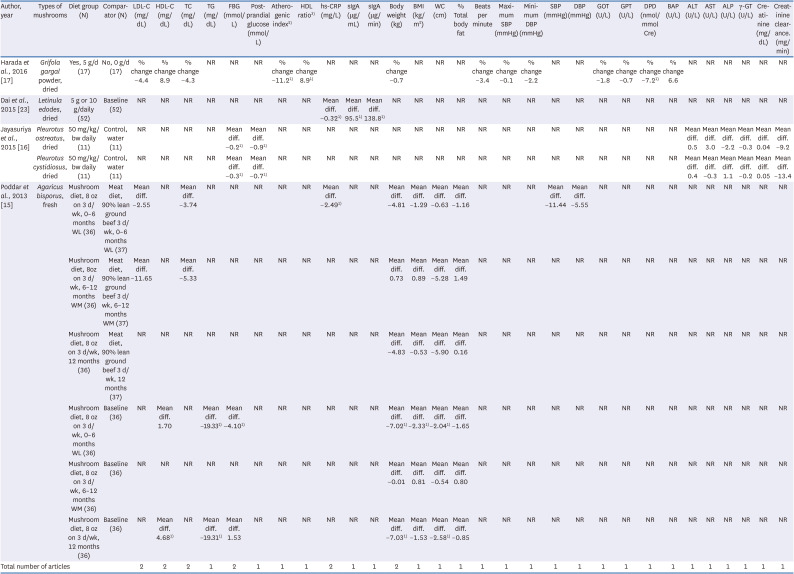
15. Poddar KH, Ames M, Hsin-Jen C, Feeney MJ, Wang Y, Cheskin LJ. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite. 2013; 71:379–387. PMID:
24056209.
16. Jayasuriya WJ, Wanigatunge CA, Fernando GH, Abeytunga DT, Suresh TS. Hypoglycaemic activity of culinary
Pleurotus ostreatus and
P. cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action. Phytother Res. 2015; 29:303–309. PMID:
25382404.
17. Harada E, Morizono T, Sumiya T, Kawagishi H. Effect of the medicinal mushroom,
Grifola gargal (Agaricomycetes), on bone turnover markers and serum lipids in middle-aged and elderly Japanese women. Int J Med Mushrooms. 2016; 18:1–7. PMID:
27279439.
18. Cheraghi Z, Mirmiran P, Mansournia MA, Moslehi N, Khalili D, Nedjat S. The association between nutritional exposures and metabolic syndrome in the Tehran Lipid and Glucose Study (TLGS): a cohort study. Public Health. 2016; 140:163–171. PMID:
27498945.
19. Lee DH, Yang M, Giovannucci EL, Sun Q, Chavarro JE. Mushroom consumption, biomarkers, and risk of cardiovascular disease and type 2 diabetes: a prospective cohort study of US women and men. Am J Clin Nutr. 2019; 110:666–674. PMID:
31172167.
20. Otsuka R, Tange C, Nishita Y, Kato Y, Tomida M, Imai T, Ando F, Shimokata H. Dietary diversity and all-cause and cause-specific mortality in Japanese community-dwelling older adults. Nutrients. 2020; 12:1052. PMID:
32290256.
21. Zhang T, Rayamajhi S, Meng G, Zhang Q, Liu L, Wu H, Gu Y, Wang Y, Zhang S, Wang X, et al. Edible mushroom consumption and incident hyperuricemia: results from the TCLSIH cohort study. Food Funct. 2021; 12:9178–9187. PMID:
34606546.
22. Ba DM, Gao X, Muscat J, Al-Shaar L, Chinchilli V, Zhang X, Ssentongo P, Beelman RB, Richie JP Jr. Association of mushroom consumption with all-cause and cause-specific mortality among American adults: prospective cohort study findings from NHANES III. Nutr J. 2021; 20:38. PMID:
33888143.
23. Dai X, Stanilka JM, Rowe CA, Esteves EA, Nieves C Jr, Spaiser SJ, Christman MC, Langkamp-Henken B, Percival SS. Consuming
Lentinula edodes (Shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J Am Coll Nutr. 2015; 34:478–487. PMID:
25866155.
24. Pounis G, Costanzo S, Persichillo M, de Curtis A, Sieri S, Vinceti M, Zito F, Di Castelnuovo AF, Donati MB, de Gaetano G, et al. Mushroom and dietary selenium intakes in relation to fasting glucose levels in a free-living Italian adult population: the Moli-sani Project. Diabetes Metab. 2014; 40:34–42. PMID:
24183901.
25. Food and Agriculture Organization. Total Production of Mushrooms and Truffles Worldwide from 2012 to 2022 (in Million Metric tons)*. New York (NY): Statista;2023.
26. SkyQuest Technology. Global Mushroom Market Regional Insights. Sanand: SkyQuest Technology;2023.
27. Ba DM, Ssentongo P, Beelman RB, Muscat J, Gao X, Richie JP Jr. Higher mushroom consumption is associated with lower risk of cancer: a systematic review and meta-analysis of observational studies. Adv Nutr. 2021; 12:1691–1704. PMID:
33724299.
28. Li J, Zou L, Chen W, Zhu B, Shen N, Ke J, Lou J, Song R, Zhong R, Miao X. Dietary mushroom intake may reduce the risk of breast cancer: evidence from a meta-analysis of observational studies. PLoS One. 2014; 9:e93437. PMID:
24691133.
29. Shahin L, Patel KM, Heydari MK, Kesselman MM. Hyperuricemia and cardiovascular risk. Cureus. 2021; 13:e14855. PMID:
34104597.
30. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013; 25:210–216. PMID:
23370374.
31. Jakše B, Jakše B, Pajek M, Pajek J. Uric acid and plant-based nutrition. Nutrients. 2019; 11:1736. PMID:
31357560.
32. Yu S, Wu X, Ferguson M, Simmen RC, Cleves MA, Simmen FA, Fang N. Diets containing shiitake mushroom reduce serum lipids and serum lipophilic antioxidant capacity in rats. J Nutr. 2016; 146:2491–2496. PMID:
27798348.
33. Petryn TS, Nagalievska MR, Wasser SP, Sybirna NO. Effect of the Lingzi or Reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes) on hyperglycemia and dyslipidemia with experimental metabolic syndrome. Int J Med Mushrooms. 2023; 25:17–30.
34. García-Cordero J, Mateos R, González-Rámila S, Seguido MA, Sierra-Cinos JL, Sarriá B, Bravo L. Dietary supplements containing oat beta-glucan and/or green coffee (poly)phenols showed limited effect in modulating cardiometabolic risk biomarkers in overweight/obese patients without a lifestyle intervention. Nutrients. 2023; 15:2223. PMID:
37432380.
35. Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr Diab Rep. 2021; 21:15. PMID:
33742318.
36. Gallego P, Luque-Sierra A, Falcon G, Carbonero P, Grande L, Bautista JD, Martín F, Del Campo JA. White button mushroom extracts modulate hepatic fibrosis progression, inflammation, and oxidative stress
in vitro and in
LDLR-/- mice. Foods. 2021; 10:1788. PMID:
34441565.
37. Arunachalam K, Sreeja PS, Yang X. The antioxidant properties of mushroom polysaccharides can potentially mitigate oxidative stress, beta-cell dysfunction and insulin resistance. Front Pharmacol. 2022; 13:874474. PMID:
35600869.
38. Sima P, Vannucci L, Vetvicka V. β-glucans and cholesterol. [review]. Int J Mol Med. 2018; 41:1799–1808. PMID:
29393350.
39. Feeney MJ, Dwyer J, Hasler-Lewis CM, Milner JA, Noakes M, Rowe S, Wach M, Beelman RB, Caldwell J, Cantorna MT, et al. Mushrooms and Health Summit proceedings. J Nutr. 2014; 144:1128S–36S. PMID:
24812070.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download