1. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013; 11:117. PMID:
23635324.
2. Valanti EK, Dalakoura-Karagkouni K, Siasos G, Kardassis D, Eliopoulos AG, Sanoudou D. Advances in biological therapies for dyslipidemias and atherosclerosis. Metabolism. 2021; 116:154461. PMID:
33290761.
3. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001; 104:503–516. PMID:
11239408.
4. Soliman GA. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 2018; 10:780. PMID:
29914176.
5. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013; 40:195–211. PMID:
23402469.
6. Ohashi R, Mu H, Wang X, Yao Q, Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM. 2005; 98:845–856. PMID:
16258026.
7. Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF Jr, Neufeld ED, Remaley AT, Fredrickson DS, et al. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci U S A. 2002; 99:407–412. PMID:
11752403.
8. Rohatgi A. Reverse cholesterol transport and atherosclerosis. Arterioscler Thromb Vasc Biol. 2019; 39:2–4. PMID:
30586333.
9. Getz GS, Reardon CA. Apoprotein E and reverse cholesterol transport. Int J Mol Sci. 2018; 19:3479. PMID:
30404132.
10. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017; 16:s27–s42. PMID:
29080338.
11. Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006; 113:2548–2555. PMID:
16735689.
12. Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003; 23:1178–1184. PMID:
12738681.
13. Dallinga-Thie GM, Dullaart RP, van Tol A. Concerted actions of cholesteryl ester transfer protein and phospholipid transfer protein in type 2 diabetes: effects of apolipoproteins. Curr Opin Lipidol. 2007; 18:251–257. PMID:
17495597.
14. Shrestha S, Wu BJ, Guiney L, Barter PJ, Rye KA. Cholesteryl ester transfer protein and its inhibitors. J Lipid Res. 2018; 59:772–783. PMID:
29487091.
15. Luo Y, Shelly L, Sand T, Reidich B, Chang G, MacDougall M, Peakman MC, Jiang XC. Pharmacologic inhibition of phospholipid transfer protein activity reduces apolipoprotein-B secretion from hepatocytes. J Pharmacol Exp Ther. 2010; 332:1100–1106. PMID:
19933370.
16. Dove DE, Linton MF, Fazio S. ApoE-mediated cholesterol efflux from macrophages: separation of autocrine and paracrine effects. Am J Physiol Cell Physiol. 2005; 288:C586–C592. PMID:
15509658.
17. Oppi S, Lüscher TF, Stein S. Mouse models for atherosclerosis research-which is my line? Front Cardiovasc Med. 2019; 6:46. PMID:
31032262.
18. Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med (Berl). 2005; 83:329–342. PMID:
15827760.
19. Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004; 24:1006–1014. PMID:
15087308.
20. Su YR, Ishiguro H, Major AS, Dove DE, Zhang W, Hasty AH, Babaev VR, Linton MF, Fazio S. Macrophage apolipoprotein A-I expression protects against atherosclerosis in ApoE-deficient mice and up-regulates ABC transporters. Mol Ther. 2003; 8:576–583. PMID:
14529830.
21. Derosa G, Maffioli P, Sahebkar A. Ellagic acid and its role in chronic diseases. Adv Exp Med Biol. 2016; 928:473–479. PMID:
27671829.
22. Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010; 31:513–539. PMID:
20837052.
23. Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010; 52:1290–1300. PMID:
20692795.
24. Park SH, Kim JL, Lee ES, Han SY, Gong JH, Kang MK, Kang YH. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr. 2011; 141:1931–1937. PMID:
21940512.
25. Khateeb J, Gantman A, Kreitenberg AJ, Aviram M, Fuhrman B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: a role for PPAR-gamma pathway. Atherosclerosis. 2010; 208:119–125. PMID:
19783251.
26. Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014; 289:24020–24029. PMID:
25074931.
27. Zaiou M, Arnold KS, Newhouse YM, Innerarity TL, Weisgraber KH, Segall ML, Phillips MC, Lund-Katz S. Apolipoprotein E;-low density lipoprotein receptor interaction. Influences of basic residue and amphipathic α-helix organization in the ligand. J Lipid Res. 2000; 41:1087–1095. PMID:
10884290.
28. Zhang SH, Reddick RL, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994; 94:937–945. PMID:
8083379.
29. Penson PE, Banach M. Natural compounds as anti-atherogenic agents: clinical evidence for improved cardiovascular outcomes. Atherosclerosis. 2021; 316:58–65. PMID:
33340999.
30. Sedighi M, Bahmani M, Asgary S, Beyranvand F, Rafieian-Kopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci. 2017; 22:30. PMID:
28461816.
31. Li RL, Wang LY, Liu S, Duan HX, Zhang Q, Zhang T, Peng W, Huang Y, Wu C. Natural flavonoids derived from fruits are potential agents against atherosclerosis. Front Nutr. 2022; 9:862277. PMID:
35399657.
32. Lutz M, Fuentes E, Ávila F, Alarcón M, Palomo I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules. 2019; 24:366. PMID:
30669612.
33. Ríos JL, Giner RM, Marín M, Recio MC. A pharmacological update of ellagic acid. Planta Med. 2018; 84:1068–1093. PMID:
29847844.
34. Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport: basic mechanisms and their roles in vascular health and disease. Circ Res. 2019; 124:1505–1518. PMID:
31071007.
35. Shen WJ, Azhar S, Kraemer FB. SR-B1: a unique multifunctional receptor for cholesterol influx and efflux. Annu Rev Physiol. 2018; 80:95–116. PMID:
29125794.
36. Mardones P, Quiñones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S, et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001; 42:170–180. PMID:
11181745.
37. Wang Z, Chen J, Zeng Z, Zhang Q, Du G, Guo X, Wei Y. The LOX-1 receptor ectopically expressed in the liver alleviates atherosclerosis by clearing Ox-LDL from the circulation. Mol Med. 2022; 28:26. PMID:
35236285.
38. Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, Tansey T, Amar MJ, Fruchart-Najib J, Duverger N, et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003; 44:296–302. PMID:
12576511.
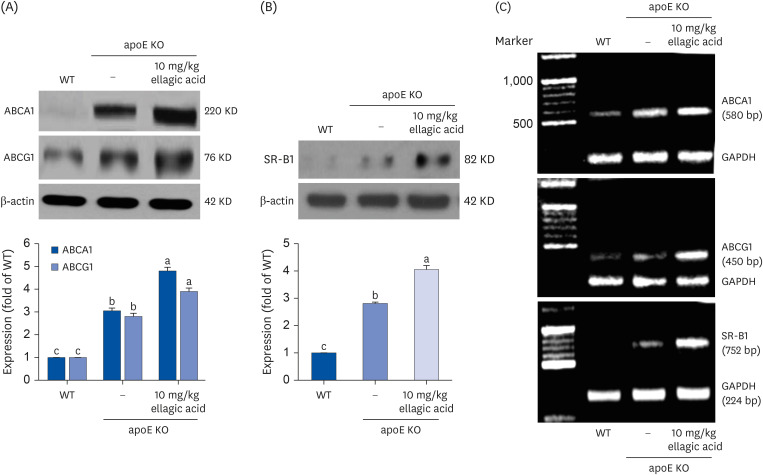
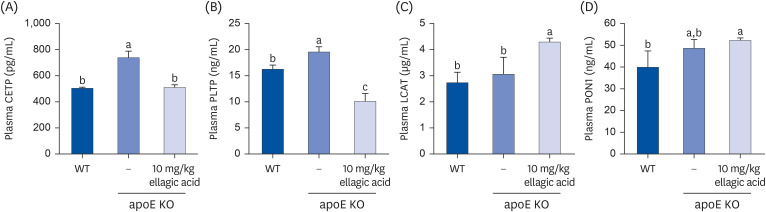
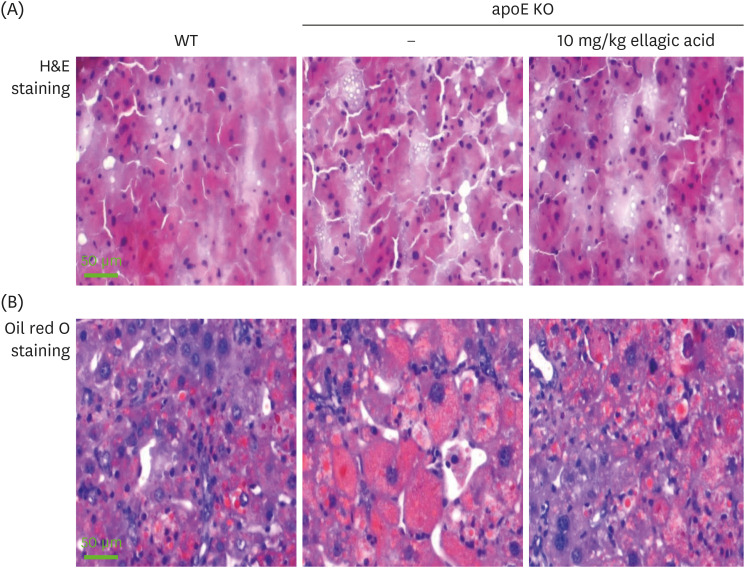
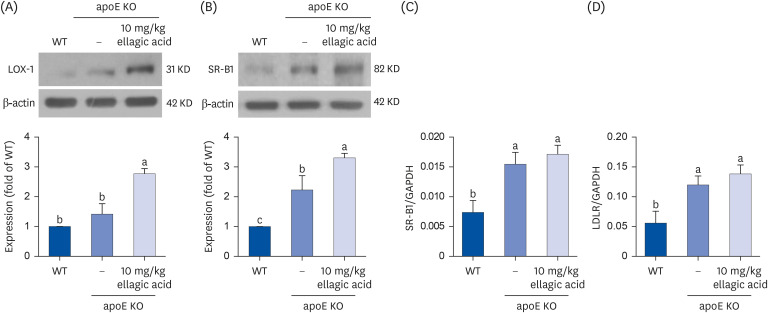




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download