1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209–249. PMID:
33538338.

2. Khil H, Kim SM, Hong S, Gil HM, Cheon E, Lee DH, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep. 2021; 11(1):2413. PMID:
33510236.

4. Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015; 1(1):15065. PMID:
27189416.

6. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129(2):422–428. PMID:
16083699.

7. Ministry of Health and Welfare (KR). National Cancer Center (KR). Quality of Guidelines of Colorectal Cancer Screening: Fecal Occult Blood Test, Colonoscopy, Double Contrast Barium Enema and Pathologic Diagnosis, Secondary Revision. Goyang, Korea: National Cancer Center;2018.
8. Meklin J, SyrjÄnen K, Eskelinen M. Fecal occult blood tests in colorectal cancer screening: systematic revies and meta-analysis of traditional and new-generation fecal immunochemical tests. Anticancer Res. 2020; 40(7):3591–3604. PMID:
32620599.

9. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019; 68(12):2179–2185. PMID:
31488504.

10. Sohn D, Kim M, Park Y, Suh M, Shin A, Lee H, et al. The Korean guideline for colorectal cancer screening. J Korean Med Assoc. 2015; 58(5):420–432.

11. Ren Y, Zhao M, Zhou D, Xing Q, Gong F, Tang W. Cost-effectiveness analysis of colonoscopy and fecal immunochemical testing for colorectal cancer screening in China. Front Public Health. 2022; 10:952378. PMID:
36033786.

12. Li XP, Chen HM, Lei XH, Dou GS, Chen YC, Chen LP, et al. Cost-effectiveness analysis of a community-based colorectal cancer screening program in Shanghai, China. J Dig Dis. 2021; 22(8):452–462. PMID:
34086400.

13. Hassan C, Benamouzig R, Spada C, Ponchon T, Zullo A, Saurin JC, et al. Cost effectiveness and projected national impact of colorectal cancer screening in France. Endoscopy. 2011; 43(9):780–793. PMID:
21623557.

14. Lew JB, St John DJ, Xu XM, Greuter MJ, Caruana M, Cenin DR, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. 2017; 2(7):e331–e340. PMID:
29253458.

15. Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016; 111(8):1092–1101. PMID:
27296945.

16. Ladabaum U, Alvarez-Osorio L, Rösch T, Brueggenjuergen B. Cost-effectiveness of colorectal cancer screening in Germany: current endoscopic and fecal testing strategies versus plasma methylated Septin 9 DNA. Endosc Int Open. 2014; 2(2):E96–104. PMID:
26135268.

17. Han D, Park J, Yun H, Bae S. Cost-effectiveness analysis of colon cancer screening by colonoscopic examination in Korea. Korean J Gastrointest Endosc. 2004; 28(1):1–8.
18. Park S, Chang Y, Yun Y, Yoo T, Huh B, Kwon S. Cost-effectiveness analysis of colorectal cancer screening in Korean general population. J Korean Acad Fam Med. 2004; 15(25):297–306.
19. Lee JY, Ock M, Jo MW, Son WS, Lee HJ, Kim SH, et al. Estimating utility weights and quality-adjusted life year loss for colorectal cancer-related health states in Korea. Sci Rep. 2017; 7(1):5571. PMID:
28717246.

20. Foundation for Industry Corporation, University of Ulsan. Estimation of Quality Adjusted Life Years Loss due to Major Cancers and Economic Evaluation of Cancer Screening Programs. Sejong, Korea: Ministry of Health and Welfare;2018.
21. Ahn J, Kim Y, Shin S, Park J. Asian Collaborative Research Project to Determine Willingness-to-Pay per Quality-Adjusted Life Year: WTP/QALY. Seoul, Korea: National Evidence-based healthcare Collaborating Agency;2012.
22. Park SM, Yun YH, Kwon S. Feasible economic strategies to improve screening compliance for colorectal cancer in Korea. World J Gastroenterol. 2005; 11(11):1587–1593. PMID:
15786532.

23. Park SM, Kim SY, Earle CC, Jeong SY, Yun YH. What is the most cost-effective strategy to screen for second primary colorectal cancers in male cancer survivors in Korea? World J Gastroenterol. 2009; 15(25):3153–3160. PMID:
19575496.

24. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999; 91(5):434–437. PMID:
10070942.

25. Lieberman DA, Weiss DG. Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001; 345(8):555–560. PMID:
11529208.

26. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013; 369(12):1106–1114. PMID:
24047060.

27. Ladabaum U, Mannalithara A, Meester RG, Gupta S, Schoen RE. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology. 2019; 157(1):137–148. PMID:
30930021.

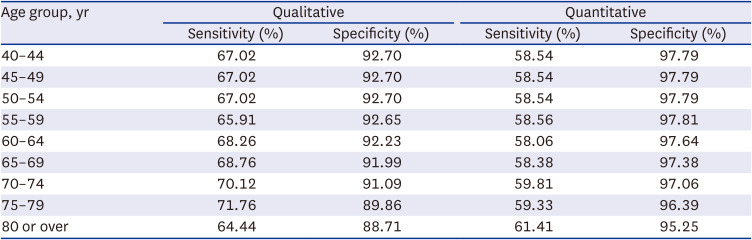
28. Park MJ, Choi KS, Lee YK, Jun JK, Lee HY. A comparison of qualitative and quantitative fecal immunochemical tests in the Korean national colorectal cancer screening program. Scand J Gastroenterol. 2012; 47(4):461–466. PMID:
22428929.

29. Huang Y, Li Q, Ge W, Cai S, Zhang S, Zheng S. Predictive power of quantitative and qualitative fecal immunochemical tests for hemoglobin in population screening for colorectal neoplasm. Eur J Cancer Prev. 2014; 23(1):27–34. PMID:
23942476.

30. Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998; 13(4):397–409. PMID:
10178664.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download