Abstract
Background
Methods
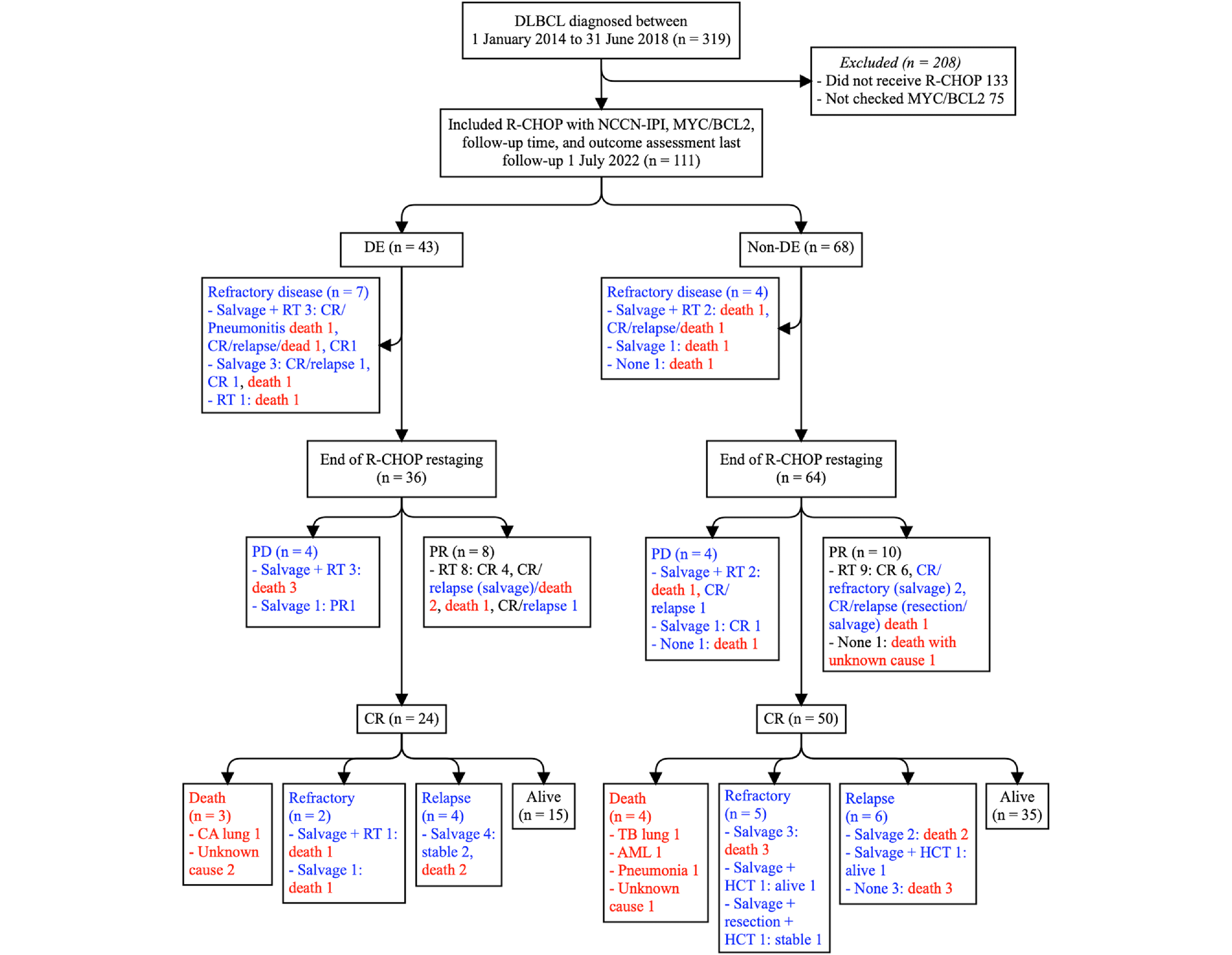
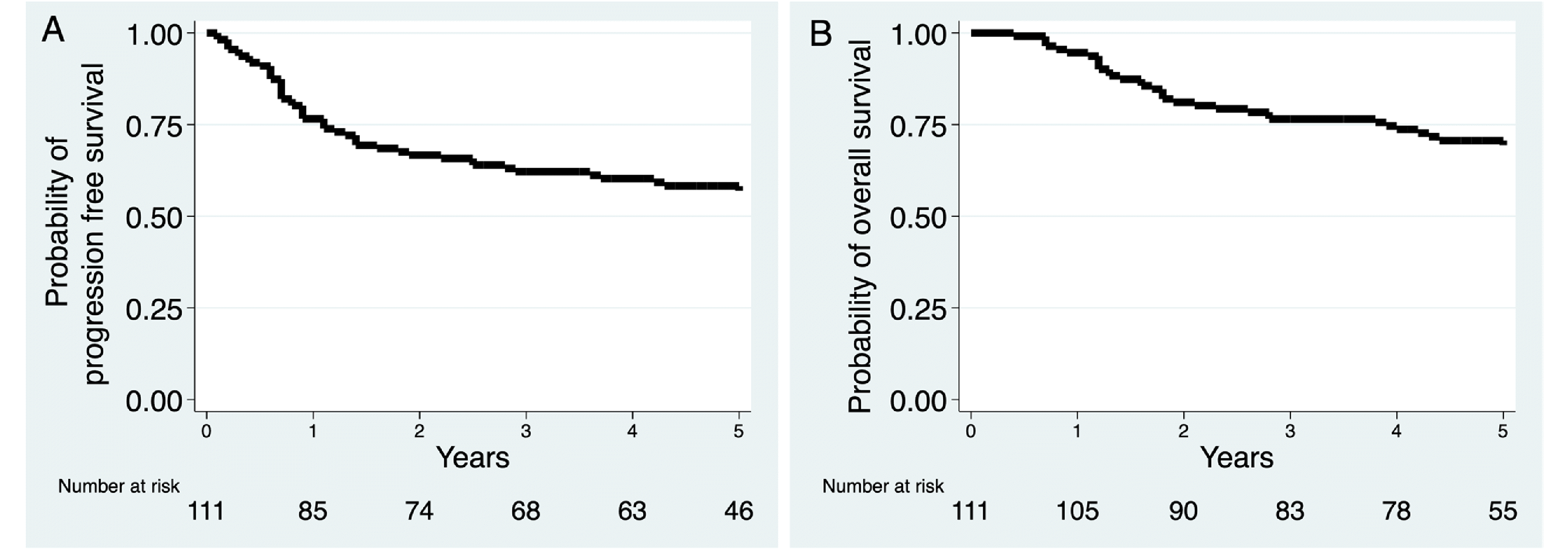
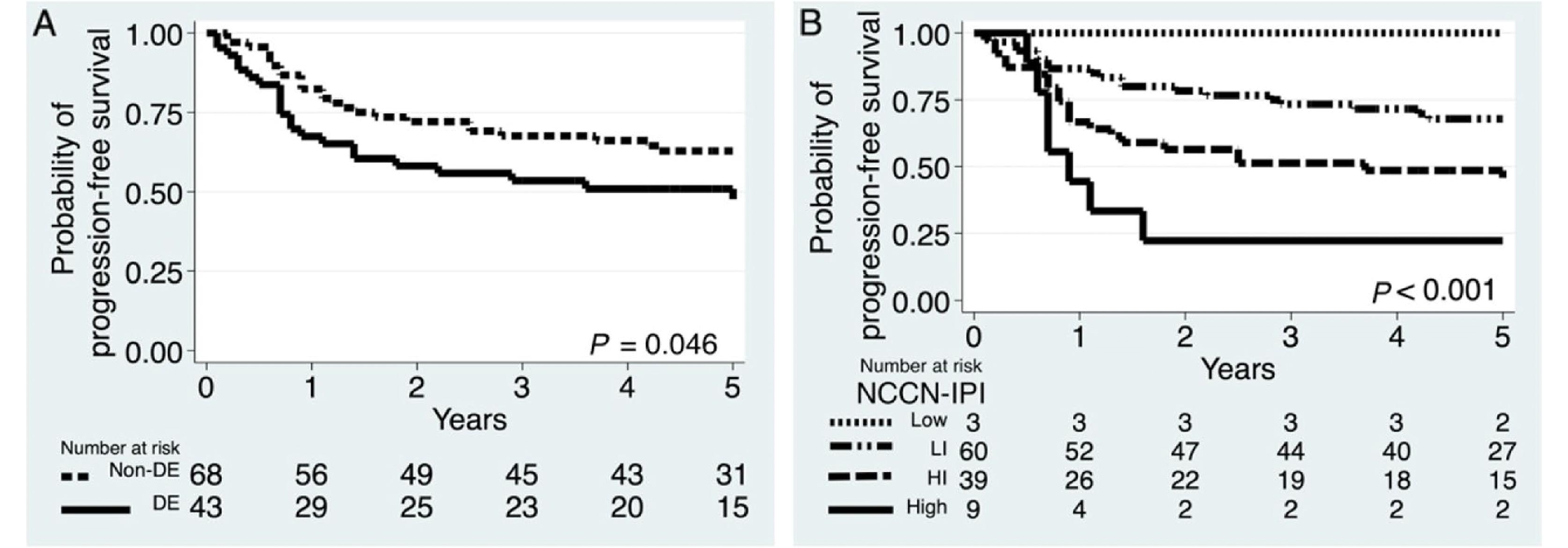
Results
Acknowledgements
Notes
Authors’ contributions
Conceptualization: N Warnnissorn, P Niparuck, N Kanitsap, and P Kulalert. Supervision: P Niparuck, N Kanitsap, P Kulalert, and S Chuncharunee. Methodology: N Warnnissorn. Software: N Warnnissorn. Resource: P Niparuck, S Chuncharunee, T Puavilai, P Chantrathammachart, S Saengboon, and L Bhoopat. Validation: N Warnnissorn. Formal analysis: N Warnnissorn. Investigation: P Boonsakan, C Suriyonplengsaeng, and N Warnnissorn. Data curation: N Warnnissorn. Writing original draft preparation: Naree Warnnissorn. Wrightreview & editing: W Limvorapitak, C Suriyonplengsaeng, and P Kulalert. Project administration: N Warnnissorn. Final approval of manuscript: All authors.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committees of Mahidol University (Faculty of Medicine, Ramathibodi Hospital; Ramathibodi MURA2018/85, dated March 23, 2018) and Thammasat University No.1 (Faculty of Medicine: Thammasat No. COA 055/2561, dated February 25, 2018); there was no intervention other than routine clinical practice, and no more than minimal risk involved. The need for informed consent was waived for this observational retrospective study.
Author details
1 Department of Pathology, Faculty of Medicine, Thammasat University, Pathumthani, Thailand. 2 Division of Hematology, Department of Medicine, Faculty of Medicine, Thammasat University, Pathumthani, Thailand. 3 Division of Hematology, Department of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. 4 Department of Pathology, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. 5 Department of Clinical Epidemiology, Faculty of Medicine, Thammasat University, Pathumthani, Thailand. 6 Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand.
REFERENCES
Table 1
Table 2
| Variables | Number (%) | 5-y PFS (95% CI) | P |
|---|---|---|---|
| Non-DE | 43 (39) | 60 (46–71) | 0.046 |
| DE | 68 (61) | 47 (31–62) | |
| NCCN-IPI Low | 3 (3) | 100 (.-.) | < 0.001 |
| LI | 60 (54) | 64 (50–75) | |
| HI | 39 (35) | 46 (30–61) | |
| High | 9 (8) | 22 (3–51) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download