INTRODUCTION
Graft-versus-host disease (GVHD) is a relatively rare complication in liver transplantation (LT), with reported incidences ranging from 0.5% to 2% [
1,
2]. However, despite aggressive treatment, mortality rates in patients with GVHD after LT can reach as high as 85% [
3,
4]. Given the high fatality rate of GVHD, previous studies have primarily focused on identifying risk factors. These include an age discrepancy of 20 years, induction with basiliximab, and the presence of hepatocellular carcinoma (HCC) [
1,
5]. GVHD develops in donor lymphocytes that are transplanted along with the liver graft [
6,
7]. Consequently, infection of donor T cells with human T cell lymphotropic virus type-1 has emerged as a significant risk factor [
8]. While CD2-blockers and TNF-α antagonists have been suggested as potential treatment options, no confirmed treatment for GVHD currently exists [
9-
11]. Therefore, the most crucial approach remains risk avoidance [
1].
Previous literature has indicated a significantly higher risk of GVHD when there is a one-way mismatch (OWMM) between the donor, who is human leukocyte antigen (HLA)-homozygous, and the recipient, who is HLA-heterozygous [
12-
15]. This risk is particularly pronounced in the case of three-loci OWMM in living donor LT (LDLT) [
15]. It is theorized that when HLA-OWMM is present, the recipient is less likely to reject passenger hematopoietic cells, which allows donor T cells to interact with the host antigen-presenting cell [
16]. The incidence of GVHD in LDLT with three-loci HLA-OWMM has been reported to be as high as 57.1%, with a 5-year patient survival rate of only 15%–20% [
12,
13]. However, various studies have reported differing incidences of GVHD and risks of posttransplant mortality [
5,
13,
15]. As such, this study aimed to report the incidence and characteristics of GVHD according to HLA-OWMM status, using data from a large, single-center LT dataset.
METHODS
The study was conducted in compliance with the Declaration of Helsinki and the Declaration of Istanbul, and received approval from the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB No. 4-2023-0955). Due to the retrospective nature of the study, informed consent was not required.
Study Materials
This single-center observational study examined 1,247 patients who underwent LT at Severance Hospital, Korea, between January 2009 and March 2023. Information on baseline characteristics, GVHD, and mortality was obtained from a prospectively collected institutional LT dataset. Specific details about GVHD, including the diagnostic method, HLA results following GVHD onset, and GVHD treatment, were gathered from electronic medical records. Patients were excluded from the study if they died within 14 days post-LT (n=70), underwent concurrent solid organ transplantation (n=18), received LT from two living donors (n=1), had a retransplantation (n=17), received an ABO-incompatible LT (n=189), had liver cancers other than HCC (n=2), or had incomplete data for HLA-A, B, or DR types (n=51). After these exclusions, 899 eligible patients were included in the study.
Definition and Outcomes
Serological typing of HLA-A, B, and DR was performed on both recipients and donors to assess donor compatibility upon admission, prior to LT. The term HLA-OWMM was used to describe a situation where one or more of the recipient's three loci (HLA-A, B, or DR) were absent in the donor's HLA, yet all of the donor's HLA loci were present in the recipient's HLA. HLA-OWMM was further classified into OWMM at one to two loci and at three loci. During the follow-up period, GVHD was suspected if the patient exhibited more than one of the following symptoms: unexplained fever, skin rash, cytopenia, and diarrhea. The diagnosis was primarily confirmed through skin biopsy, but in some instances, donor HLA chimerism (the presence of donor HLA in the recipient's peripheral blood) or a colonoscopic biopsy was used. The baseline characteristics, incidence of GVHD, and patient survival rates were then compared between the HLA-OWMM and control groups.
Human Leukocyte Antigen Serotyping Method
The diversity observed in HLA serotyping techniques can be attributed to the collection of data over an extended period. The initial step involved HLA serotyping of HLA-A, HLA-B, and HLA-DRB1 loci using the AVITA plus HLA SBT kit (BioWithus Inc.) on an ABI PRISM 3500xL Genetic analyzer (Applied Biosystems). The sequences were then analyzed using the BIOWITHUS SBT Analyzer (BioWithus Inc.) [
17]. Starting from 2022, the All Type NGS Assay kit (One Lambda Inc.) has been in use. The library was sequenced using the Illumina NextSeq technology platform (Illumina). The sequences were then analyzed using the TypeStream Visual NGS analysis program (ver. 2.1.0.40; One Lambda Inc.) and the IPD-IMGT/HLA Database (ver. 3.40.0.1) [
18].
Immunosuppression
For the majority of patients, the immunosuppressant regimen began with induction immunosuppression using basiliximab. This was administered at a dosage of 20 mg on days 0 and 4 post-LT. Following this, maintenance immunosuppression was introduced, which included a regimen featuring a calcineurin inhibitor, specifically tacrolimus, used in combination with prednisolone and mycophenolate mofetil (MMF). Patients were initially given an intravenous dose of methylprednisolone, ranging from 500 to 1,000 mg. This dosage was systematically decreased over a predetermined period until the administration was transitioned to oral prednisolone, which was maintained at a daily dose of 5–10 mg. However, for patients diagnosed with HCC, a different approach was taken. In these instances, the use of MMF was replaced with an mammalian target of rapamycin inhibitor, which was initiated 1 month post-LT.
Statistical Methods
Data are reported as number (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. The baseline characteristics were compared between the HLA-OWMM and control groups using the chi-square test or Wilcoxon rank-sum test, if applicable. Kaplan-Meier curves with the log-rank test were used to analyze survival between the groups. Cox proportional hazard regression models were used to evaluate the association between HLA-OWMM and time-to-GVHD outcomes. A sensitivity analysis was conducted using propensity score matching to validate the results of this study. Patients with HLA-OWMM were matched to control group patients at a ratio of 1:5 using the nearest neighbor matching algorithm. The covariates were recipient age, recipient sex, the year of LT, HCC, pretransplant Model for End-Stage Liver Disease (MELD) score, donor age, and donor sex. The caliper used in matching was 0.2. All analyses were performed using the R ver. 4.2.0 (R Foundation), with the threshold for significance set at P<0.05.
RESULTS
Out of 899 LT patients, 14 (1.6%) exhibited HLA-OWMM, as shown in
Table 1. Six of these patients demonstrated HLA-OWMM at one to two loci, while the remaining eight showed HLA-OWMM at three loci. The group included three pediatric patients, aged 0, 1, and 10 years. Only two patients underwent deceased donor LT (DDLT), with the remaining 12 receiving LDLT. The majority of living donors (n=10) were the patients' offspring, and two were the patients' parents. Within the HLA-OWMM group, two patients (14.3%) developed GVHD. Including these two patients with GVHD, three patients (21.4%) died within 1 year following LT.
Table 2 provides detailed information on 15 LT recipients who developed GVHD. Unfortunately, GVHD was a significant contributing factor to an 80% mortality rate, affecting 12 out of the 15 patients. Two of these patients had HLA-OWMM. The diagnostic data indicates a median onset time of GVHD of 17 days posttransplantation, with an IQR of 16–20 days. Diagnosis was primarily confirmed through skin biopsy. In our study, all patients received steroid pulse therapy as the initial treatment intervention, and seven patients (46.7%) discontinued their immunosuppressants.
In
Table 3, the baseline characteristics of the HLA-OWMM group and the control group are compared. The two groups showed similar age (52 years [IQR, 40–61 years in the HLA-OWMM group] vs. 54 years [IQR, 47–59 years in the control group]; P=0.456), sex (female in 28.6% vs. 30.4%; P=0.989) and body mass index (22.8 kg/m
2 [IQR, 20.8–23.8 kg/m
2] vs. 23.5 kg/m
2 [IQR, 21.3–25.8 kg/m
2]; P=0.326). Among the underlying liver diseases, hepatitis C (14.3% vs. 6.8%) and biliary atresia (14.3% vs. 6.0%) were more frequent, while alcoholic liver disease was less frequent in the HLA-OWMM group than in the control group, although these differences were not statistically significant (P=0.677). HCC was also numerically more frequent in the HLA-OWMM group, but without statistical significance (64.5% vs. 41.4%, P=0.379). The pretransplant MELD score was significantly lower in the HLA-OWMM group than in the control group (11 [IQR, 8–15] vs. 15 [IQR, 10–25]; P=0.013]. In the HLA-OWMM group, 14.3% of donors were deceased, compared to 32.9% in the control group (P=0.057). The HLA-OWMM group was younger than the control group, with marginal significance (28 years [IQR, 25–34 years] vs. 35 years [IQR, 26–47 years]; P=0.083). Through Kaplan-Meier analysis, we identified a significant difference in the occurrence of GVHD between the HLA-OWMM group and the control group (
Supplementary Fig. 1). Furthermore, HLA-OWMM was independently associated with a higher incidence of GVHD (
Supplementary Table 1).
When recipients were divided according to donor type, the overall incidence of GVHD was 1.3% (8/606) in LDLT recipients and 2.4% (7/293) in DDLT recipients (
Table 4). LDLT recipients with HLA-OWMM at one to three loci had a 16.7% (2/12) incidence of GVHD, which was significantly higher than that in LDLT recipients without HLA-OWMM (1.0% [6/594], P=0.001). GVHD was not observed in DDLT recipients with HLA-OWMM.
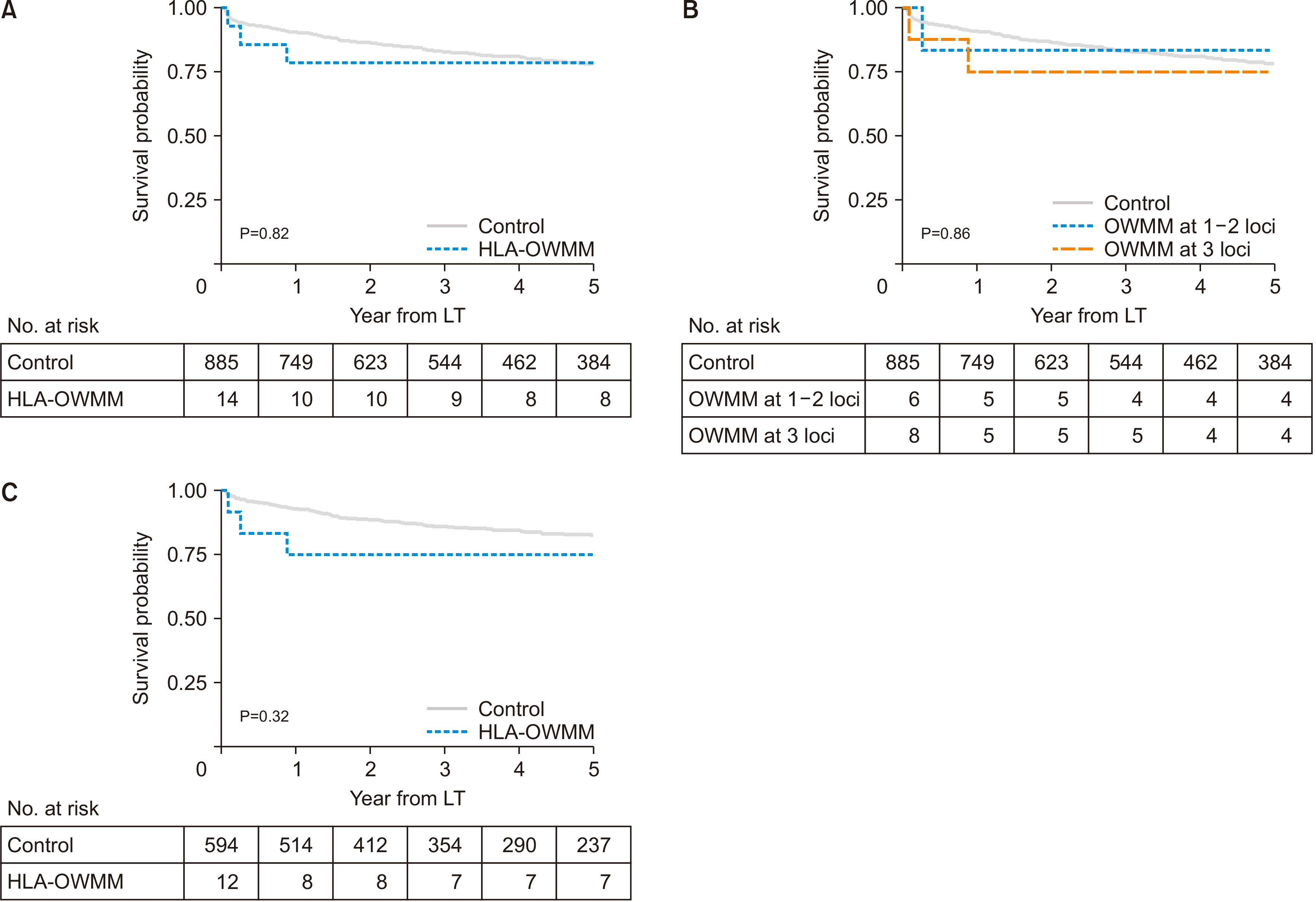
The survival rate was 78.6% at both 1 and 5 years in the HLA-OWMM group and 90.7% and 78.2% at 1 and 5 years after LT, respectively, in the control group (P=0.821) (
Fig. 1). When the HLA-OWMM group was stratified by the number of mismatched loci, patients with OWMM at one to two loci showed a 5-year survival rate of 83.3%, whereas those with OWMM at three loci had a rate of 75.0% (P=0.865). When compared exclusively within LDLT recipients, the survival rate in the HLA-OWMM group was 75.0% at both 1 and 5 years, while in the control group, it was 93.0% and 82.6% at 1 and 5 years after LT, respectively (P=0.328).
Propensity score matching was conducted to reduce selection bias and balance the variables between the HLA-OWMM and control groups (
Supplementary Table 2,
Supplementary Fig. 2). We confirmed that even after propensity score matching, the 5-year patient survival rates in the HLA-OWMM and control groups remained similar (P=0.960) (
Supplementary Fig. 3).
DISCUSSION
This study demonstrated a higher incidence of GVHD in LT recipients with donor-to-recipient HLA-OWMM. The incidence of GVHD was significantly higher in LDLT recipients when HLA-OWMM was present, whereas no association was observed between HLA-OWMM and GVHD in DDLT recipients. Despite the higher incidence of GVHD, patients with HLA-OWMM, including those with OWMM at three loci, exhibited similar 5-year survival rates to the control patients in our cohort.
Previous studies have reported that the incidence of GVHD in LDLT patients with OWMM at three loci is >50%, and the 5-year patient survival rate is only 15%–20% [
12-
15]. Nevertheless, the incidence of GVHD in patients with LDLT in this study was 16.7% (both HLA-OWMM at one to two loci and at three loci), corresponding to approximately one-third of the rates reported in previous studies. Furthermore, the 5-year survival rate in LDLT patients with OWMM at three loci was 75%, which was more than three times higher than that reported in previous studies. Although the exact reason is unknown, this discrepancy may be attributed to ethnic differences and variations in immunosuppressant protocols among institutions. A distinctive aspect of this report, distinguishing it from other studies, is that nearly all LT recipients and donors (95.5%) in our dataset underwent preoperative HLA typing and OWMM was assessed. This could at least partially explain the difference in findings between this and previous studies. Furthermore, it is worth noting that this study had only six LDLT recipients with HLA-OWMM at three loci. The limited number of cases could be attributed to the relatively small sample size. Consequently, it may be necessary to conduct surveys involving a larger cohort of patients with HLA-OWMM.
Although this finding did not reach statistical significance, it is important to notice the age discrepancy between the HLA-OWMM group and the control group (median, 28 vs. 35 years; P=0.083). The former group primarily consisted of younger donors, attributed to the prevalence of parent-to-offspring LDLT within this group. On the contrary, DDLT recipients, typically characterized by an older donor demographic, predominated in the control group, underscoring the significant age contrast between the two groups.
In this study, we observed notable findings related to GVHD in patients with HLA-OWMM. Specifically, the incidence of GVHD was significantly higher in the HLA-OWMM group compared to the control group. This led to a minor discrepancy in the 1-year patient survival rates due to the increased mortality associated with GVHD. However, there was no significant difference in the 5-year patient survival rates between the two groups. This absence of a significant difference in the long-term survival rate could be due to the relatively low incidence of GVHD compared to previous studies.
Among the 14 patients in the HLA-OWMM group, three died within a year. One patient's death was due to causes unrelated to GVHD, while the other two were directly linked to GVHD. The first of these patients developed severe neutropenia, a condition attributed to GVHD, and subsequently died from sepsis on the 31st day posttransplantation. The second patient suffered from active bleeding in the main bronchus, a situation worsened by pancytopenia, also attributed to GVHD. Attempts to control the bleeding through fiberoptic bronchoscopy were unsuccessful, resulting in the patient's death on the 94th day posttransplantation. However, the remaining 11 patients did not develop GVHD and survived beyond a year.
The relatively high short-term mortality rate within 1 year (around 10%) could pose a challenge to performing LDLT in patients with HLA-OWMM. However, the viability of LDLT should be evaluated by comparing the survival rate of patients on the waiting list for DDLT in regions with severe organ shortages, such as South Korea [
19,
20]. This decision-making process becomes particularly critical when a patient's only available donor is a living one with an HLA-OWMM.
In addition, our findings showed that patients in the HLA-OWMM group who underwent LDLT had survival rates similar to those in the control group. A multivariable logistic regression analysis suggested that LDLT was not independently linked to a higher incidence of GVHD. These results suggest that HLA-OWMM should not be considered an absolute contraindication for LDLT, especially in cases of organ shortage.
Despite this, HLA-OWMM was found to be associated with a higher rate of GVHD in recipients of LDLT as compared to those who underwent DDLT. Previous studies focusing on DDLT have identified recipient age and HCC as risk factors for GVHD, but no reports have suggested HLA-mismatch as a risk factor [
5]. Unlike in DDLT, the influence of HLA-OWMM in LDLT from a related donor is determined by chromosome zygosity rather than the HLA genotype itself [
13,
21]. Therefore, other genetic characteristics may also contribute to the development of GVHD, indicating a need for further research in this area.
Furthermore, it is essential to acknowledge the influence of immunosuppressive agents on the risk of mortality in GVHD. Prior research has identified a correlation between the use of basiliximab as an induction immunosuppressant and an increase in fatal outcomes in GVHD. Conversely, maintenance immunosuppression regimens that employ MMF are known to be less associated with GVHD mortality [
5]. However, the variability in the duration and dosage of MMF administration introduces complex challenges that could potentially impact the reliability and accuracy of the results. In this context, thoroughly addressing the association between MMF use and GVHD is beyond the scope of our study.
There are a few limitations of this study that should be noted. First, due to the single-center LT dataset, the absolute numbers of GVHD and HLA-OWMM cases were too small to yield accurate outcomes. The treatments of GVHD were diverse and were not standardized. However, we demonstrated that patients with HLA-OWMM showed acceptable long-term graft survival despite a higher GVHD incidence and increased short-term mortality, even in cases with HLA-OWMM at three loci.
In conclusion, patients with HLA-OWMM exhibited a higher incidence of GVHD and a rise in short-term mortality, especially for LDLT. Nevertheless, over the long term, the mortality risk attributed to GVHD diminished. The decision to exclude living donors with HLA-OWMM should be made carefully and in a nuanced manner, taking into account the specific health conditions of LT candidates and the availability of deceased donor organs in various regions.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download