Abstract
Background
As sleep disturbances are common in the intensive care unit (ICU), this study assessed the sleep quality in the ICU and identified barriers to sleep.
Methods
Patients admitted to the ICUs of a tertiary hospital between June 2022 and December 2022 who were not mechanically ventilated at enrollment were included. The quality of sleep (QoS) at home was assessed on a visual analog scale as part of an eight-item survey, while the QoS in the ICU was evaluated using the Korean version of the Richards-Campbell Sleep Questionnaire (K-RCSQ). Good QoS was defined by a score of ≥50.
Results
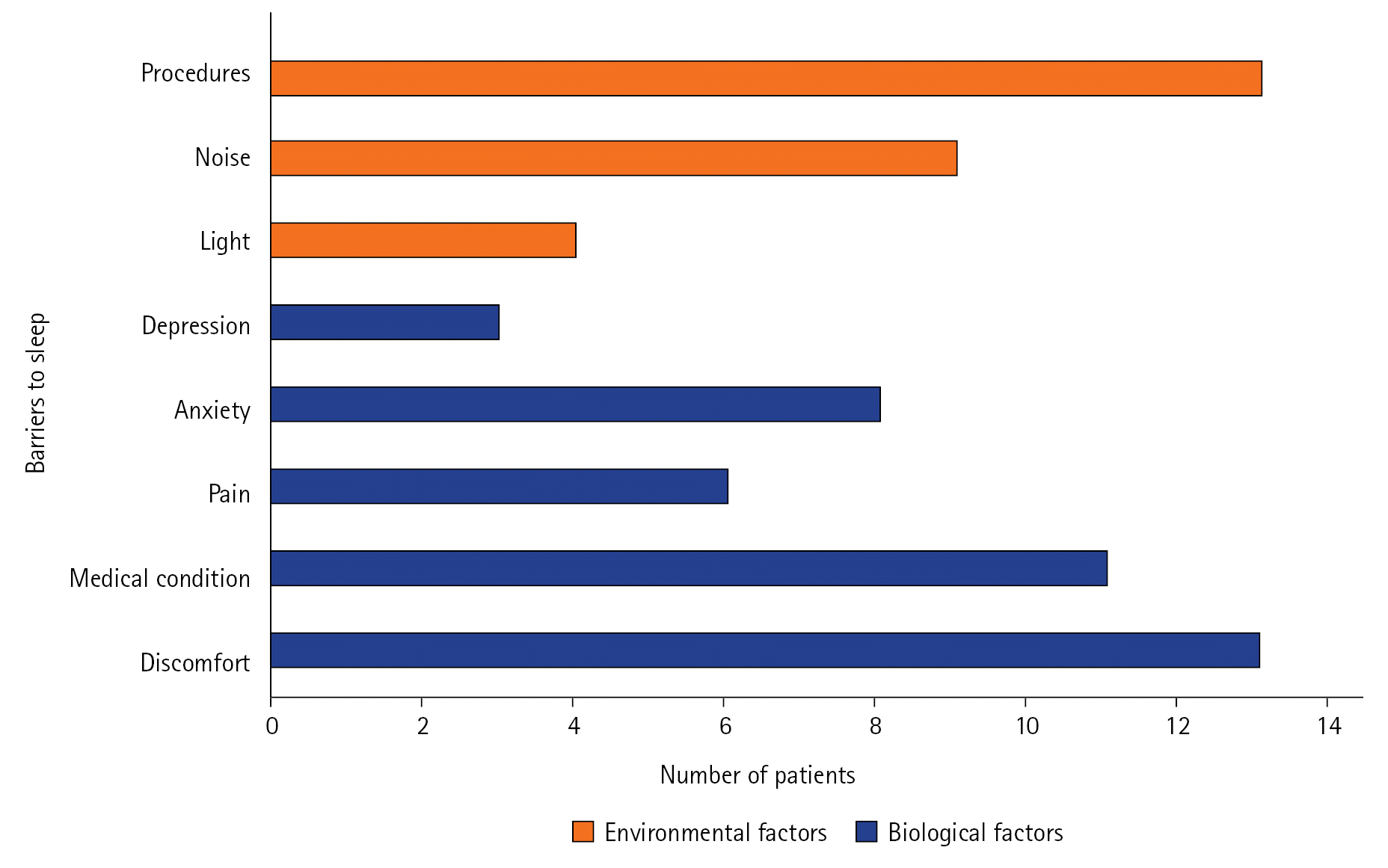
Of the 30 patients in the study, 19 reported a QoS score <50. The Spearman correlation coefficient showed no meaningful relationship between the QoS at home and the overall K-RCSQ QoS score in the ICU (r=0.16, P=0.40). The most common barriers to sleep were physical discomfort (43%), being awoken for procedures (43%), and feeling unwell (37%); environmental factors including noise (30%) and light (13%) were also identified sources of sleep disruption. Physical discomfort (median [interquartile range]: 32 [28.0–38.0] vs. 69 [42.0–80.0], P=0.004), being awoken for procedures (36 [20.0–48.0] vs. 54 [36.0–80.0], P=0.04), and feeling unwell (31 [18.0–42.0] vs. 54 [40.0–76.0], P=0.01) were associated with lower K-RCSQ scores.
Humans spend approximately one-third of the day sleeping, and sufficient sleep is essential for productivity and overall well-being [1]. Abnormal sleep is associated with numerous adverse consequences, including cognitive impairment [2], depression [3], and mortality [4]. Studies have also shown bidirectional associations between sleep deprivation and delirium, with poor sleep triggering delirium and delirium contributing to further sleep disruption [5,6]. Sleep is largely orchestrated by two mechanisms: a circadian system that maintains periodicity and a neural network that ensures sleep adequacy [7]. In critically ill patients, both mechanisms are disturbed by a combination of factors, including the exposure to various sedatives, stressful nature of the intensive care unit (ICU) environment, and physiological effects of acute illness [7]. As inadequate sleep is associated with poor outcomes, it is important to investigate the sleep quality of patients in the ICU and to understand the factors associated with poor quality of sleep (QoS) during critical illness.
Subjective assessments of sleep quality using questionnaires are an alternative to objective measurements that are often difficult to perform in the ICU. The Richards-Campbell Sleep Questionnaire (RCSQ) consists of five items rated on a visual analog scale that measure five domains of sleep: sleep latency, efficiency, depth, number of awakenings, and overall sleep quality [8]. The Korean version of the RCSQ (K-RCSQ) has shown high reliability for assessing sleep quality among ICU patients [9], and this tool can be readily utilized at the bedside. Although the gold standard for objective sleep measurement is the polysomnography, subjective assessments including the K-RCSQ offer valid insight into the patients’ overall sleep experience at lower cost and greater accessibility. Additionally, subjective evaluations can be repeated during the patient's stay in the ICU, and questionnaires can easily be used for daily assessments as well as for follow-up after interventions.
The sleep disturbances that critically ill patients experience in the ICU can be attributed to several factors. Haimovich et al. [10] showed that the circadian clock gene expression in peripheral blood leukocytes was altered during acute inflammation, suggesting that the inflammatory response observed during critical illness can disrupt the circadian rhythm. Environmental factors, including noise and light, have also been identified as barriers to sleep [7,11-13]. To improve sleep quality in the ICU, we must assess the QoS of critically ill patients and identify potentially modifiable factors influencing sleep. Although a study confirming the reliability of the K-RCSQ was previously conducted, studies on the real-world applications of the K-RCSQ are lacking. Thus, this study aimed to subjectively assess the overall sleep quality of patients using the K-RCSQ and to identify factors associated with sleep disturbances in the ICU.
This single-center, descriptive pilot study analyzed adult patients admitted to the medical ICUs of Seoul National University Hospital between June 2022 and December 2022. All but five among the 29 beds of the ICUs were single-patient rooms. Patients aged ≥19 years who were not mechanically ventilated at the time of enrollment were eligible to participate. The level of arousal was measured using the Richmond Agitation-Sedation Scale (RASS), a 10-point scale that uses positive values to denote different levels of anxiety or agitation, zero to denote a calm and alert state, and negative values to denote different levels of sedation [14]. Patients with RASS scores ranging from −2 to +2 were considered to have adequate mental capacities to participate in the study. After patients had spent more than 24 hours in the ICU, the study process was explained to those who were able to follow commands and make autonomous decisions; for all study participants, informed consent was obtained by the attending physician. As a pilot study, a convenience sample of 30 patients was surveyed, and the response rate was 100%. All data were anonymized to ensure individual privacy, and this study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2204-167-1321). Informed consent was obtained from all participants.
The first part of the survey consisted of eight questions that evaluated the QoS prior to ICU admission and baseline levels of anxiety and depression (Supplementary Figure 1). Patients were asked to rate their QoS at home on a visual analog scale ranging from 0 (worst possible sleep) to 100 (best possible sleep). They were also asked to indicate whether they slept regularly at home, had ever experienced symptoms of insomnia, duration of sleep, average bedtimes, and medications used for insomnia (if any) prior to hospitalization. Three questions adopted from the Hospital Anxiety and Depression Scale [15] were used to assess the levels of anxiety and depression among patients.
The second part of the survey was conducted after patients had spent more than 24 hours in the ICU. Patients were asked to rate their QoS using the five K-RCSQ items and to identify any barriers to sleep in the ICU (Supplementary Figure 2). A “good” QoS was a priori defined as a score ≥50 on the visual analog scale, which has previously shown high sensitivity and specificity in determining good QoS [16,17].
The QoS scores on the visual analogue scale were treated as non-normally distributed continuous data. Patients were categorized into two groups based on overall K-RCSQ scores: the good QoS group included patients with a score ≥50, while the poor QoS group included those with an overall score <50. Categorical variables were expressed as counts and percentages, and continuous variables were expressed as means and standard deviations (SDs) or medians and interquartile ranges (IQRs). Between-group differences were assessed using the Mann-Whitney U-test for quantitative variables and Fisher’s exact test for qualitative variables. The Spearman rank-correlation method was used to compare the QoS at home with the overall K-RCSQ QoS score in the ICU. As a pilot study, the sample size was not calculated a priori [18-20]. All analyses were two-tailed, and a P-value <0.05 was considered significant. Statistical analyses were performed using the IBM SPSS ver. 24.0 (IBM Corp.).
Thirty patients completed the survey during the study period, 19 of whom had an overall QoS score <50 in the ICU. The baseline characteristics of the study population are summarized in Table 1. The mean age was 59±14 years, 16 (53%) were men, and the median ICU length of stay was 6.5 days (IQR, 4.0–10.8 days). The median Sequential Organ Failure Assessment (SOFA) score and clinical frailty score were 6 (4–9.8) and 4 (4–5), respectively. Overall, 16 patients (53%) required MV, and 12 patients (40%) received continuous renal replacement therapy during their ICU stay. Although patients in the poor sleep group had a higher prevalence of congestive heart failure (10 [52.6%] vs. 1 [9.1%], P=0.02), no significant differences were observed in the incidence of other comorbidities. Regarding medications used within 24 hours prior to the survey, 19 (63%) patients received treatment with opioids, 16 (53.3%) with steroids, 18 (60%) with dexmedetomidine, and four (13.3%) with benzodiazepines. No differences in the use of medications between the two groups were observed. Of the 18 patients who received dexmedetomidine, six (20%) were under light sedation the night before and at the time of the survey.
Of the 12 (40%) patients who had experienced insomnia prior to ICU admission, nine (75%) had used medications to treat their insomnia. No significant difference in the number of patients with insomnia before ICU admission was seen between the two groups (9 [47.4%] vs. 3 [27.3%], P=0.44) (Table 1). The self-reported QoS scores of the study participants are presented in Table 2. Patients reported a median of 7 hours (6–8 hours) of sleep at home and a median bedtime of 10 PM (9–11 PM). In addition, 18 patients (60%) reported sufficient sleep at home and had a median QoS score of 60 (36.3–73.8). The Spearman correlation coefficient showed no meaningful relationship between QoS at home and the overall K-RCSQ QoS score in the ICU (r=0.16, P=0.40). The mean (SD) scores for each K-RCSQ item were as follows: 48.3 (32.3) for sleep depth, 49.5 (31.4) for falling asleep, 50.5 (30.7) for awakening, 51.7 (28.4) for returning to sleep, and 48.3 (31.3) for overall sleep quality (Supplementary Table 1).
The self-reported factors influencing the QoS in the ICU are illustrated in Figure 1. The most common barriers to sleep were physical discomfort (43%), being awoken for procedures (43%), and feeling unwell due to underlying medical conditions (37%). Environmental factors including noise (30%) and light (13%) were also identified as sources of sleep disruption. The median K-RCSQ scores for each sleep barrier are presented in Table 3. Physical discomfort (median [IQR]: 32 [28.0–38.0] vs. 69 [42.0–80.0], P=0.004), being awoken for procedures (36 [20.0–48.0] vs. 54 [36.0–80.0], P=0.04), and feeling unwell (31 [18.0–42.0] vs. 54 [40.0–76.0], P=0.01) were significantly associated with lower overall K-RCSQ scores (Table 3). When each factor influencing sleep was compared between patients with good QoS and those with poor QoS, no significant differences were observed (Table 4).
This descriptive pilot study showed that the QoS at home was not significantly correlated with the QoS in the ICU and identified possible factors influencing sleep quality among critically ill patients. We found that physical discomfort, being awoken for procedures, and feeling unwell were significantly associated with lower K-RCSQ scores; the identification of such factors is the first step toward improving the QoS in critically ill patients. Additionally, this study was the first to use the K-RCSQ to evaluate sleep quality in a specific cohort of patients admitted to medical ICUs. As sleep disturbance can lead to adverse physical and psychological consequences, it is important to understand how well patients are sleeping and to identify potentially modifiable barriers to sleep in the ICU.
Our study showed that the QoS in the ICU did not differ from that at home, which conflicts with the results of a previous study of 56 critically ill patients that showed significantly worse QoS in the ICU than prior to hospitalization (median, 7.15/10; Z=−3.02; P=0.002) [21]. In another study of 144 hospitalized patients, the mean sleep duration in the hospital was significantly lower than that at home (5.7 hours vs. 7.1 hours, P <0.01) [11]. Such conflicting results can perhaps be attributed to heterogeneity within the patient population and differences in hospital environments: in comparison with multiple occupancy rooms, stays in single-patient rooms have been associated with a faster onset of rapid-eye-movement sleep [22]. While most patients included in our study stayed in single-patient rooms, more than half of the patients in the study by Delaney et al. [11] stayed in multiple-occupancy rooms—such environmental differences may have contributed to the discrepancies observed between our study and existing literature..
Patients in our study reported that environmental factors, including light and sound, were barriers to sleep in the ICU. The average sound levels in the ICU are between 41–68 dB [21,23,24], exceeding the World Health Organization recommendations of 30 dB at night [25]. Moreover, sound is a common source of sleep disturbance [13,21], and noise in the ICU can potentially be reduced without much compromise for patient safety. An observational study comparing sound levels in different ICU rooms showed that 64% of disruptive sounds were caused by monitor alarms and conversations unrelated to patient care [26]. Such noise can be reduced by minimizing redundant alarms and avoiding unnecessary dialogue during nighttime hours. In addition to sound, light has also been reported as a source of sleep disturbance [12,13], and approximately 13% of patients in the present study reported light as a significant barrier to sleep in the ICU. Even short- and low-intensity light levels have been associated with circadian misalignment [13], and brief flashes of light can cause larger circadian phase shifts compared with continuous light [27]. The use of shades, dimming of lights, and application of blindfolds may be simple measures to reduce the negative impacts of light on sleep in the ICU.
Approximately 43% of patients reported that physical discomfort and being awoken for procedures negatively influenced sleep quality in the ICU. Noxious stimuli such as frequent position changes and suctioning can be bothersome, and physical discomfort has previously been recognized as being detrimental to sleep in critically ill patients [28,29]. Gabor et al. [23] reported that patient care interactions occur up to eight times for every hour of sleep, and activities such as blood draws, vital sign measurements, and dressing changes at night can be disruptive. Simple measures such as delaying non-essential procedures until the morning and minimizing the number of position changes at night may be helpful in improving the QoS of patients in the ICU. Although optimizing the ICU environment for better sleep is important, such interventions must be performed carefully to avoid compromising patient safety and overall quality of care.
Our study has several strengths. This prospective study identified modifiable factors associated with poor sleep quality in the ICU. By recognizing such factors, potential measures can be taken to optimize the ICU environment to allow for better sleep. Additionally, our study used the K-RCSQ to assess sleep quality in a noninvasive manner and showed that the K-RCSQ is a valid, readily available tool for measuring QoS in critically ill patients at the bedside. Taken together, the results of our study not only showcase the QoS of patients in the ICU, but also provide insight into the possible areas of change that can translate to better sleep and, ultimately, to better patient outcomes.
Despite its strengths, our study has some limitations. First, this was a single-center study that included a limited number of patients, which restricts the generalizability of our findings. Second, as our study used subjective assessments of sleep, recall bias can be a source of confounding. Third, six patients received dexmedetomidine the night before the survey, which may have affected their responses to the questionnaire. The effects of dexmedetomidine have been shown to resemble natural sleep [30], and its sedative and analgesic properties may have influenced the sleep quality of patients who were under light sedation at the time of the survey. Fourth, as this study did not conduct follow-up evaluations of sleep quality throughout a patient’s time in the ICU, our results do not reflect the changes in the QoS over the entire ICU stay. In addition, we were unable to gather information on sleep duration in the ICU, which further limits the comprehensiveness of the study. Finally, as different tools were used to assess QoS at home and in the ICU, making comparisons between the two is limited; further validation studies are needed to assess the usefulness of the K-RCSQ for evaluating the QoS at home.
Among critically ill patients, the QoS at home was not significantly correlated with the QoS in the ICU. Noise, light, patient care interactions, physical discomfort, and feeling unwell were identified as possible barriers to sleep. Many of the barriers identified in this study are potentially modifiable, and further studies are needed to investigate whether modification of such factors is associated with improvement of sleep quality in the ICU.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2023.00514.
Supplementary Figure 1.
Survey of quality of sleep prior to intensive care unit admission.
acc-2023-00514-Supplementary-Fig-1.pdf
Supplementary Figure 2.
Korean Version of the Richards-Campbell Sleep Questionnaire and Survey of Barriers to Sleep in the intensive care unit.
acc-2023-00514-Supplementary-Fig-2.pdf
REFERENCES
1. Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008; 9:910–9.

2. Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017; 18:404–18.

3. Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020; 45:74–89.

4. Parthasarathy S, Vasquez MM, Halonen M, Bootzin R, Quan SF, Martinez FD, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015; 128:268–75.
5. Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009; 35:781–95.

6. Pisani MA, D’Ambrosio C. Sleep and delirium in adults who are critically ill: a contemporary review. Chest. 2020; 157:977–84.

8. Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000; 8:131–44.

9. Kim JK, Park JH, Cho J, Lee SM, Lee J. Reliability of the Korean version of the Richards-Campbell Sleep Questionnaire. Acute Crit Care. 2020; 35:164–8.

10. Haimovich B, Calvano J, Haimovich AD, Calvano SE, Coyle SM, Lowry SF. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010; 38:751–8.

11. Delaney LJ, Currie MJ, Huang HC, Lopez V, Van Haren F. “They can rest at home”: an observational study of patients’ quality of sleep in an Australian hospital. BMC Health Serv Res. 2018; 18:524.

12. Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S. A patient survey of sleep quality in the intensive care unit. Minerva Anestesiol. 2012; 78:406–14.

13. Martinez FE, Poulter AL, Seneviratne C, Chrimes A, Havill K, Balogh ZJ, et al. ICU patients’ perception of sleep and modifiable versus non-modifiable factors that affect it: a prospective observational study. J Clin Med. 2022; 11:3725.

14. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–44.
15. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–70.

16. Naik RD, Gupta K, Soneja M, Elavarasi A, Sreenivas V, Sinha S. Sleep quality and quantity in intensive care unit patients: a cross-sectional study. Indian J Crit Care Med. 2018; 22:408–14.

17. Locihová H, Axmann K, Žiaková K, Šerková D, Černochová S. Sleep quality assessment in intensive care: actigraphy vs. Richards-Campbell sleep questionnaire. Sleep Sci. 2020; 13:235–41.
18. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharma Stat. 2005; 4:287–91.

20. Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013; 13:104.

21. Stewart JA, Green C, Stewart J, Tiruvoipati R. Factors influencing quality of sleep among non-mechanically ventilated patients in the Intensive Care Unit. Aust Crit Care. 2017; 30:85–90.

22. Van Eijk M, Slooter A. Quality and quantity of sleep in multipatient versus single-room ICUs. Crit Care. 2012; 16(Suppl 1):P321.

23. Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003; 167:708–15.

24. Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013; 17:R187.

25. Berglund B, Lindvall T, Schwela DH; World Health Organization. Guidelines for community noise. World Health Organization; 1999.
26. Tegnestedt C, Günther A, Reichard A, Bjurström R, Alvarsson J, Martling CR, et al. Levels and sources of sound in the intensive care unit: an observational study of three room types. Acta Anaesthesiol Scand. 2013; 57:1041–50.

27. Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl). 2019; 23:147–56.

28. Bihari S, Doug McEvoy R, Matheson E, Kim S, Woodman RJ, Bersten AD. Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012; 8:301–7.

Table 1.
Characteristics of the patients according to the quality of sleep in the ICU
| Characteristic | Poor quality of sleep (n=19) | Good quality of sleep (n=11) | P-value |
|---|---|---|---|
| Age (yr) | 60±15 | 58±11 | 0.68a) |
| Male | 12 (63.2) | 4 (36.4) | 0.26 |
| Body mass index (kg/m2) | 21.2±4.19 | 23.6±3.5 | 0.12a) |
| Comorbidity | |||
| Cardiovascular disease | 5 (26.3) | 0 | 0.13 |
| Congestive heart failure | 10 (52.6) | 1 (9.1) | 0.02 |
| Chronic obstructive pulmonary disease | 5 (26.3) | 1 (9.1) | 0.37 |
| Diabetes mellitus | 7 (36.8) | 3 (27.3) | 0.70 |
| Chronic liver disease | 4 (21.1) | 4 (36.4) | 0.42 |
| Chronic kidney disease | 1 (12.5) | 6 (54.5) | 0.16 |
| Solid tumor malignancy | 3 (15.8) | 3 (27.3) | 0.64 |
| Leukemia | 1 (5.3) | 3 (27.3) | 0.13 |
| Clinical frailty scaleb) | 5 (4.0–5.5) | 4 (3.5–4.5) | 0.14a) |
| Insomnia prior to ICU, yes | 9 (47.4) | 3 (27.3) | 0.44 |
| Insomnia medication prior to ICU, yes | 7 (36.8) | 2 (18.2) | 0.42 |
| SOFA scorec) | 6 (4.0–9.5) | 7 (4.0–9.5) | 0.71a) |
| ICU length of stay (day) | 6.5 (4.0–10.8) | 7 (6.5–9.0) | 0.46a) |
| Survey date (day) | 4 (2–34) | 3 (2–10) | 0.66a) |
| MV during ICU stay, yes | 10 (52.6) | 6 (54.5) | 1.0 |
| CRRT during ICU stay, yes | 8 (42.1) | 4 (36.4) | 1.0 |
| Reason for ICU admission | |||
| Respiratory | 12 (63.2) | 8 (72.7) | 0.70 |
| Cardiovascular | 7 (36.8) | 1 (9.1) | 0.20 |
| Renal replacement therapy | 7 (36.8) | 3 (27.3) | 0.70 |
| Gastrointestinal bleeding | 1 (5.3) | 1 (9.1) | 1.0 |
| Neurologic | 1 (5.3) | 0 | 1.0 |
| Sepsis | 13 (68.4) | 7 (63.6) | 1.0 |
| Drugs used in the ICUd) | |||
| Steroids | 9 (47.4) | 7 (63.6) | 0.47 |
| Benzodiazepines | 3 (15.8) | 1 (9.1) | 1.0 |
| Opioids | 12 (63.2) | 7 (63.6) | 1.0 |
| Dexmedetomidine | 11 (57.9) | 5 (45.5) | 0.71 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
ICU: intensive care unit; SOFA: Sequential Organ Failure Assessment; MV: mechanical ventilation; CRRT: continuous renal replacement therapy.
Table 2.
Self-reported quality of sleep at home and in the ICU
Table 3.
K-RCSQ scores according to barriers to sleep
Table 4.
Factors influencing sleep quality in the ICU




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download