1. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016; 9(8):e002922. PMID:
27436837.
2. Elhassan MG, Chao PW, Curiel A. The conundrum of volume status assessment: revisiting current and future tools available for physicians at the bedside. Cureus. 2021; 13(5):e15253. PMID:
34188992.

3. Craig M, Pereira NL. Right heart catheterization and risk stratification in advanced heart failure. Curr Heart Fail Rep. 2006; 3(3):143–152. PMID:
16914107.

4. Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015; 28(1):40–56. PMID:
25559474.

5. Arjamaa O. Physiology of natriuretic peptides: the volume overload hypothesis revisited. World J Cardiol. 2014; 6(1):4–7. PMID:
24527182.

6. Park JH, Jo YI, Lee JH. Clinical usefulness of bioimpedance analysis for assessing volume status in patients receiving maintenance dialysis. Korean J Intern Med. 2018; 33(4):660–669. PMID:
29961308.

7. Malbrain ML, Huygh J, Dabrowski W, De Waele JJ, Staelens A, Wauters J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther. 2014; 46(5):381–391. PMID:
25432557.

8. Ciumanghel AI, Grigoras I, Siriopol D, Blaj M, Rusu DM, Grigorasi GR, et al. Bio-electrical impedance analysis for perioperative fluid evaluation in open major abdominal surgery. J Clin Monit Comput. 2020; 34(3):421–432. PMID:
31201590.

9. Mayne KJ, Shemilt R, Keane DF, Lees JS, Mark PB, Herrington WG. Bioimpedance indices of fluid overload and cardiorenal outcomes in heart failure and chronic kidney disease: a systematic review. J Card Fail. 2022; 28(11):1628–1641. PMID:
36038013.

10. Pirlich M, Schütz T, Spachos T, Ertl S, Weiss ML, Lochs H, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000; 32(6):1208–1215. PMID:
11093726.
11. Yu SJ, Kim DH, Oh DJ, Yu SH, Kang ET. Assessment of fluid shifts of body compartments using both bioimpedance analysis and blood volume monitoring. J Korean Med Sci. 2006; 21(1):75–80. PMID:
16479069.

12. Park CS, Lee SE, Cho HJ, Kim YJ, Kang HJ, Oh BH, et al. Body fluid status assessment by bio-impedance analysis in patients presenting to the emergency department with dyspnea. Korean J Intern Med. 2018; 33(5):911–921. PMID:
29241303.

13. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42(36):3599–3726. PMID:
34447992.

14. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022; 145(18):e895–1032. PMID:
35363499.
15. Ji E, Kim YS. Prevalence of chronic kidney disease defined by using CKD-EPI equation and albumin-to-creatinine ratio in the Korean adult population. Korean J Intern Med. 2016; 31(6):1120–1130. PMID:
27017386.

16. Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3(1):1–150.
17. Choi G, Yoon HJ, Song YJ, Jeong HM, Gu JE, Han M, et al. Consistency of the estimated target weights and ECW/TBW using BIA after hemodialysis in patients between standing and lying-down positions. BMC Nephrol. 2022; 23(1):106. PMID:
35300597.

18. Yang EM, Park E, Ahn YH, Choi HJ, Kang HG, Cheong HI, et al. Measurement of fluid status using bioimpedance methods in korean pediatric patients on hemodialysis. J Korean Med Sci. 2017; 32(11):1828–1834. PMID:
28960036.

19. Liu MH, Wang CH, Huang YY, Tung TH, Lee CM, Yang NI, et al. Edema index established by a segmental multifrequency bioelectrical impedance analysis provides prognostic value in acute heart failure. J Cardiovasc Med (Hagerstown). 2012; 13(5):299–306. PMID:
22367574.

20. Liu MH, Wang CH, Huang YY, Tung TH, Lee CM, Yang NI, et al. Edema index-guided disease management improves 6-month outcomes of patients with acute heart failure. Int Heart J. 2012; 53(1):11–17. PMID:
22398670.

21. Lyons KJ, Bischoff MK, Fonarow GC, Horwich TB. Noninvasive bioelectrical impedance for predicting clinical outcomes in outpatients with heart failure. Crit Pathw Cardiol. 2017; 16(1):32–36. PMID:
28195941.

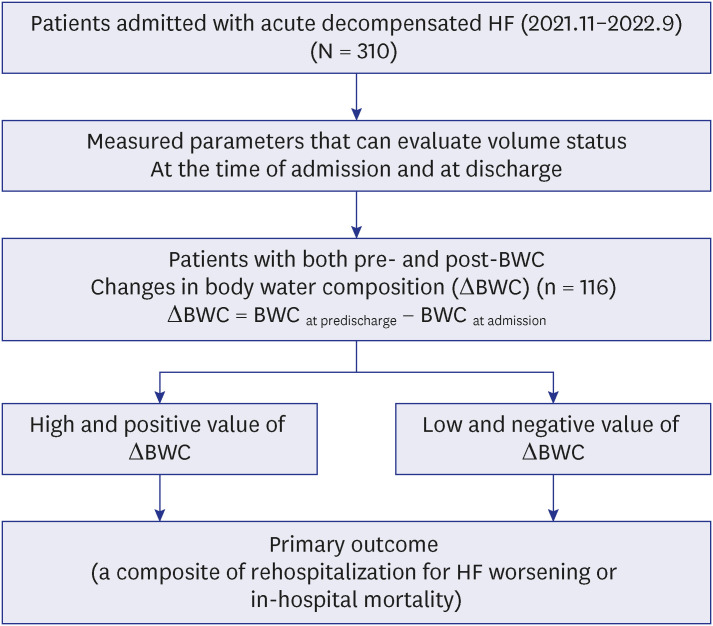
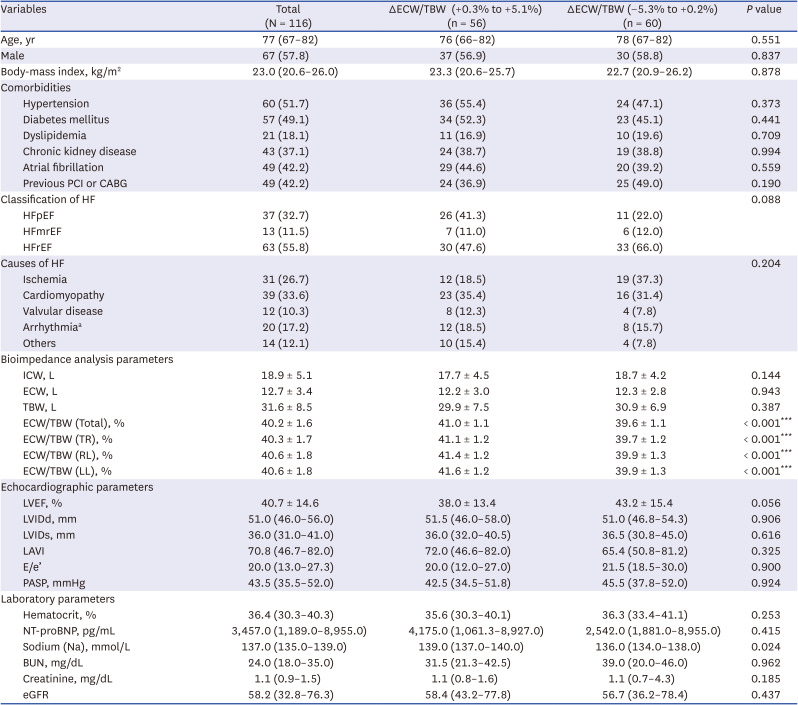
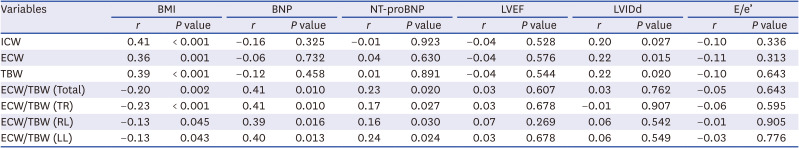
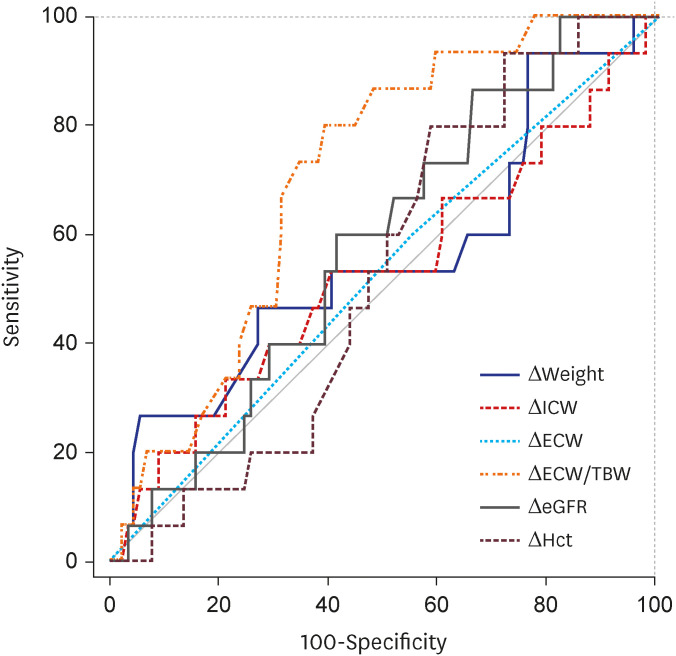




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download