Introduction
Materials and Methods
1. Patients selection
2. Treatment scheme for IMTs in FUSCC
Results
1. Patients’ characteristics
1) Clinical characteristics
Table 1
2) Molecular characteristics
2. Patients’ treatment strategy
1) Surgery
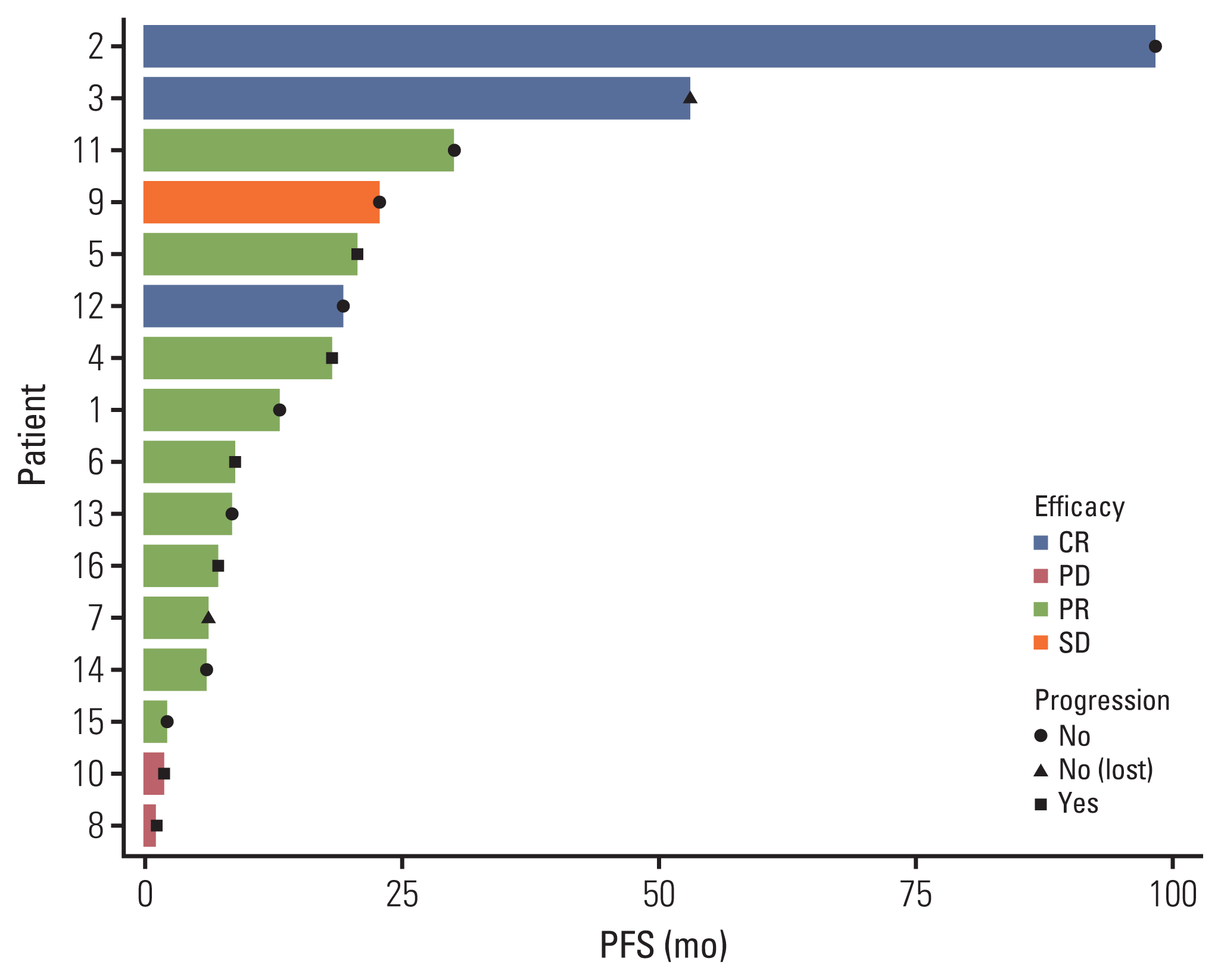
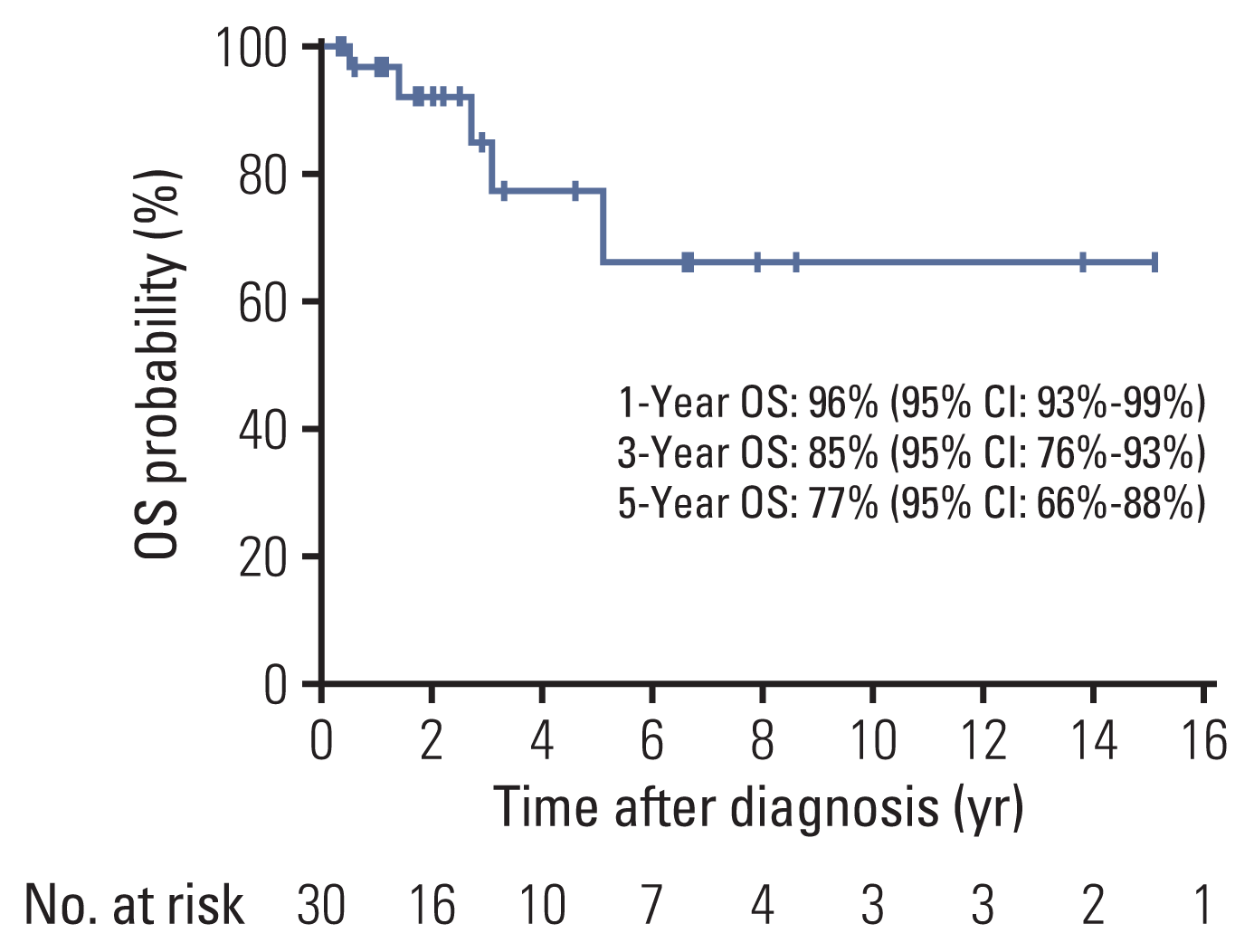
2) Systemic treatments and outcomes
Table 2
| Patient No. | Sex/Age (yr) | Primary tumor site | Metastatic sites | Radical surgery | ALK IHC/ALK FISH/NGS tests | ALK inhibitor (BOR, PFS) | Other systemic therapy (BOR) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M/34 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | −/−/No actionable alterations | No | PLD+IFO (SD) | Lost |
| 2 | F/41 | Abdominopelvic mesentery | No | Yes | −/−/NA | No | No | No relapse, alive |
| 3 | M/56 | Retroperitoneum | Abdominopelvic mesentery | Yes | + (cytoplasmic)/+/NA | No | No | Relapse, dead |
| 4 | M/50 | Lung | No | Yes | −/−/NA | No | No | No relapse, alive |
| 5 | M/51 | Lung | No | Yes | NA | No | No | No relapse, alive, had nasopharyngeal carcinoma 8 years before the diagnosis of lung IMT |
| 6 | M/58 | Abdominopelvic mesentery | Abdominopelvic mesentery | Yes | −/−/NA | No | No | Relapse, lost |
| 7 | F/51 | Lung | No | Yes | NA | No | No | No relapse, developed pancreatic adenocarcinoma (PAC) 4.3 years after the diagnosis of lung IMT and died of PAC |
| 8 | F/32 | Bladder | No | Yes | + (cytoplasmic)/+/NA | No | No | Lost |
| 9 | M/26 | Stomach | No | Yes | + (cytoplasmic)/+/NA | No | No | No relapse, alive |
| 10 | M/25 | Lung | No | Yes | + (cytoplasmic)/+/TPM3-ALK translocation | No | No | No relapse, alive |
| 11 | F/52 | Retroperitoneum | No | Yes | + (cytoplasmic)/+/NA | No | No | No relapse, alive |
| 12 | F/41 | Breast | No | Yes | NA | No | No | No relapse, alive, developed thyroid cancer and cervical cancer 4.1 years and 7.7 years after the diagnosis of breast IMT, respectively |
| 13 | M/77 | Liver | No | Yes | −/−/NA | No | No | No relapse, alive |
| 14 | F/36 | Abdominopelvic mesentery | No | Yes | + (cytoplasmic)/+/NA | No | No | No relapse, alive |
| 15 | F/22 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (cytoplasmic)/+/NA | Crizotiniba) (PR, > 13.3 mo) | No | Alive |
| 16 | F/47 | Abdominopelvic mesentery | Abdominopelvic mesentery, liver, lymph nodes | No | + (cytoplasmic)/+/NA | Crizotinibb) (CR, > 98.2 mo) | ADM (PD) | Alive, developed stage I lung adenocarcinoma 7.3 years after the diagnosis of pelvic IMT |
| 17 | M/22 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (nuclear)/+/NA | Crizotiniba) (CR, > 53.1 mo) | No | Lost |
| 18 | F/24 | Lung | Lung, pleura | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PR, 18.3 mo) | Paclitaxel+ carboplatin (PD) | Dead |
| 19 | F/74 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (nuclear)/+/NA | Crizotiniba) (PR, 20.8 mo), ceritinib (PR) | No | Lost |
| 20 | M/28 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (nuclear)/+/NA | Crizotiniba) (PR, 9.0 mo), ceritinib (PR), lorlatinib (PR) | No | Dead |
| 21 | F/25 | Abdominopelvic mesentery and lymph nodes | Liver, lung, bone, mediastinal lymph nodes | No | + (nuclear)/+/NA | Crizotiniba) (PR, > 6.3 mo) | No | Lost |
| 22 | M/39 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (nuclear)/+/NA | Crizotiniba) (PD, 1.3 mo) | No | Dead |
| 23 | F/51 | Abdominopelvic mesentery | Abdominopelvic mesentery and lymph nodes | No | + (nuclear)/+/NA | Crizotiniba) (SD, > 23.0 mo) | No | Alive |
| 24 | F/28 | Abdominopelvic mesentery | Abdominopelvic mesentery | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PD, 2.0 mo) | ADM+IFO (PD), anlotinib (SD) | Alive |
| 25 | F/21 | Abdominopelvic mesentery | Abdominopelvic mesentery and lymph nodes | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PR, > 30.2 mo) | No | Alive |
| 26 | F/56 | Abdominopelvic mesentery | Abdominopelvic mesentery | No | + (nuclear)/+/RANBP2-ALK translocation | Crizotiniba) (CR, > 19.4 mo) | No | Alive |
| 27 | F/28 | Uterus | Abdominopelvic mesentery and lymph nodes | No | + (cytoplasmic)/+/IGFBP5-ALK translocation | Crizotiniba) (PR, > 8.7 mo) | No | Alive |
| 28 | F/49 | Abdominopelvic mesentery | Abdominopelvic mesentery | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PR, > 6.1 mo) | No | Alive |
| 29 | F/51 | Lung | Abdominopelvic mesentery, mediastinal lymph nodes | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PR, > 2.3 mo) | No | Alive |
| 30 | M/28 | Rectum | Liver | Yes | + (cytoplasmic)/+/NA | Crizotiniba) (PR, 7.3 mo) | Apatinib (SD) | Alive |
ADM, doxorubicin; ALK, anaplastic lymphoma kinase; BOR, best of response, CR, complete response; F, female; FISH, fluorescence in situ hybridization; IFO, ifosfamide; IGFBP5, insulin like growth factor binding protein 5; IHC, immunohistochemical analysis; IMT, inflammatory myofibroblastic tumor; M, male; NA, not available; NGS, next-generation sequencing; PD, progressive disease; PFS, progression-free survival; PLD, pegylated-liposome doxorubicin; PR, partial response; RANBP2, RAN binding protein 2; SD, stable disease.
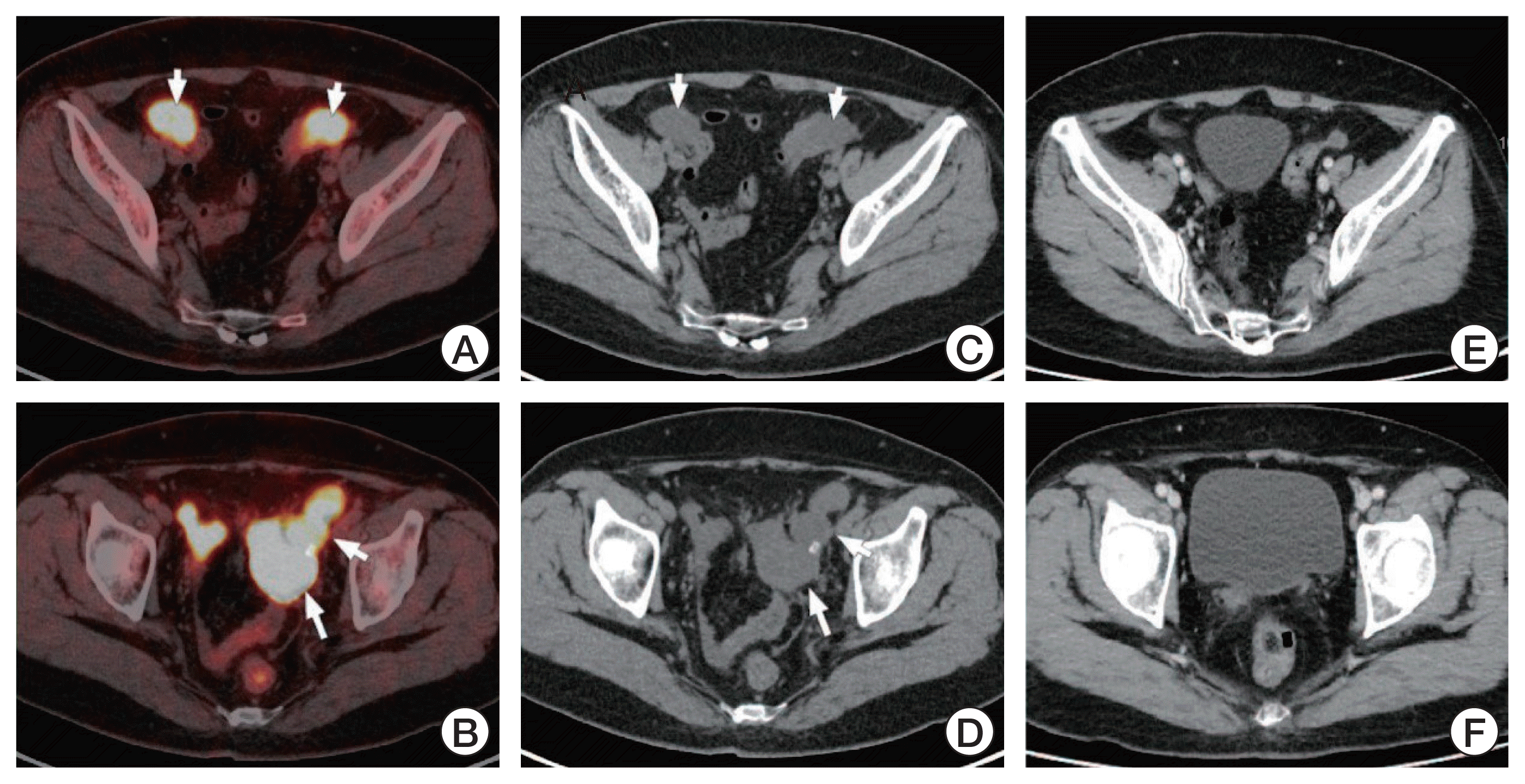
Fig. 1




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download