Abstract
Background
This study evaluated the efficacy and safety of add-on gemigliptin in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycemic control with metformin and dapagliflozin.
Methods
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study, 315 patients were randomized to receive either gemigliptin 50 mg (n=159) or placebo (n=156) with metformin and dapagliflozin for 24 weeks. After the 24-week treatment, patients who received the placebo were switched to gemigliptin, and all patients were treated with gemigliptin for an additional 28 weeks.
Results
The baseline characteristics were similar between the two groups, except for body mass index. At week 24, the least squares mean difference (standard error) in hemoglobin A1c (HbA1c) changes was –0.66% (0.07) with a 95% confidence interval of –0.80% to –0.52%, demonstrating superior HbA1c reduction in the gemigliptin group. After week 24, the HbA1c level significantly decreased in the placebo group as gemigliptin was administered, whereas the efficacy of HbA1c reduction was maintained up to week 52 in the gemigliptin group. The safety profiles were similar: the incidence rates of treatment-emergent adverse events up to week 24 were 27.67% and 29.22% in the gemigliptin and placebo groups, respectively. The safety profiles after week 24 were similar to those up to week 24 in both groups, and no new safety findings, including hypoglycemia, were noted.
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by multiple pathophysiologic abnormalities that contribute to hyperglycemia. Currently, multiple antidiabetic medications targeting the pathophysiological defects are available [1]. As T2DM progresses, monotherapy alone cannot maintain glycemic control, and combination therapy with various medications is necessary.
Sodium glucose cotransporter 2 (SGLT2) inhibitors inhibit glucose reabsorption and prompt glucose excretion in the proximal tubule of the kidney, resulting in lowered plasma glucose levels and weight loss in an insulin-independent manner. A dipeptidyl peptidase-4 (DPP-4) inhibitor inhibits the degradation of incretin, including glucagon-like peptide-1 (GLP-1). This results in an increase in GLP-1, leading to increased insulin secretion and reduced glucagon secretion, finally resulting in the lowering of both fasting and postprandial glucose with minimal risk of hypoglycemia [2-10]. Owing to the different modes of action targeting the different pathophysiologic abnormalities, the combination of SGLT2 inhibitor and DPP-4 inhibitor could suggest an additive effect on improving the glucose-lowering efficacy with a low risk of hypoglycemia. Besides additive glycemic control, a beneficial impact on weight loss is expected considering that SGLT2 inhibitors have effects on weight loss, and DPP-4 inhibitors are generally weight-neutral. Furthermore, the beneficial effects of SGLT-2 inhibitors and neutral effects of DPP-4 inhibitors on cardiovascular or renal risk/progression, proven in large clinical trials, would support this combination therapy. Therefore, the combination of SGLT2 inhibitor and DPP-4 inhibitor would result in complementary effects in improving the glucose-lowering efficacy with comparable safety profiles over placebo and good tolerability, including no increased risk of weight gain and hypoglycemia [11,12]. Moreover, addition of DDP-4 inhibitors to SGLT2 inhibitors and metformin has been evaluated in previous clinical trials [13-15].
Gemigliptin is a DPP-4 inhibitor developed by LG Chem (Seoul, Korea), which was marketed after approval by the Ministry of Food and Drug Safety in the Republic of Korea in 2012, and its efficacy and safety have been confirmed in various clinical studies [16-21]. This study aimed to evaluate and compare the efficacy and safety of gemigliptin 50 mg as an add-on therapy with placebo in patients with T2DM who had inadequate glycemic control with metformin and dapagliflozin.
This 52-week multicenter, randomized, placebo-controlled, parallel-group, double-blind, phase III study was conducted at 31 sites in the Republic of Korea from April 2019 to November 2021. After an 8-week washout period and/or a 2-week run-in period, eligible patients were randomized to receive either gemigliptin 50 mg or placebo once daily as an add-on therapy to dapagliflozin 10 mg and metformin ≥1,000 mg in a 1:1 ratio with stratification by hemoglobin A1c (HbA1c; <8.5% or ≥8.5%) and estimated glomerular filtration rate (eGFR; <90 or ≥90 mL/min/1.73 m2) for 24 weeks (main treatment period [MTP]). After week 24, the patients who received the placebo were switched to gemigliptin 50 mg until week 52 (extension treatment period [ETP]). Thus, all patients received gemigliptin 50 mg once daily with dapagliflozin and metformin from weeks 24 to 52. Therefore, the overall treatment period (OTP) was 52 weeks (Supplemental Fig. S1). In addition to the background therapy of dapagliflozin and metformin, rescue therapy was allowed when patients continued to have hyperglycemia.
Randomization was performed using the interactive web response system, and a randomization code was prepared using the stratified block randomization method, considering the pre-specified block size and two stratification factors. As this study was conducted in a double-blinded manner, both investigators and patients were unaware of the treatment group to which the patients were randomly assigned.
This study (NCT03842267) was approved by the Institutional Review Boards (Chungnam National University Hospital, 2019-02-040-019, etc.) and conducted in compliance with the International Council for Harmonization Good Clinical Practice (ICH GCP) guidelines and relevant guidelines that complied with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to participation.
This study enrolled patients aged ≥19 years who had been treated with dapagliflozin 10 mg/day+metformin ≥1,000 mg/day at a stable dose for 8 weeks and had 7%≤ HbA1c ≤11%, fasting plasma glucose (FPG) ≤270 mg/dL, and eGFR ≥45 mL/min/1.73 m2 at screening. The key exclusion criteria were body mass index (BMI) >40 kg/m2 and congestive heart failure. The detailed inclusion and exclusion criteria are listed in Supplemental Table S1.
The primary endpoint was the change in HbA1c level at week 24 from baseline. Secondary endpoints included changes in HbA1c, FPG, and fasting lipid parameters, body weight, waist circumference, eGFR, and albuminuria from baseline, percentage of patients who received rescue therapy up to week 52, and response rate of HbA1c <7% and <6.5% at weeks 24 and 52. Safety endpoints included the assessment of adverse events (AEs), clinical laboratory test results, incidence of hypoglycemia, vital signs, and electrocardiograms.
A total of 314 participants (157 per group, including a 20% dropout rate) were considered to achieve 80% power with a one-sided significance level of 2.5%, assuming an effect size of 0.39% and a standard deviation of 1.1% when gemigliptin was added to dapagliflozin and metformin.
The sample size was determined based on the results of studies of gemigliptin in T2DM-naïve patients, saxagliptin in T2DM-naïve patients, and saxagliptin as an add-on to SGLT2 inhibitor and metformin in patients with T2DM who were treated with SGLT2 inhibitor and metformin [13,16,22].
All randomized patients who had been administered the investigational product (placebo or gemigliptin) at least once were included in the safety set for the safety analyses. All randomized patients who had been administered the investigational product at least once and had HbA1c levels measured at baseline and at least once after were included in the full analysis set, following the intention-to-treat principle for efficacy analyses.
The primary efficacy endpoint was analyzed using the mixed-effect model, with HbA1c change as a dependent variable, treatment, baseline HbA1c, baseline eGFR, visit, and treatment-by-visit interaction as fixed effects, and patient as a random effect. For the HbA1c responder rate, the adjusted response rate was analyzed using logistic regression with baseline HbA1c and eGFR as covariates. Two-sided 95% confidence intervals (CIs) for least squares mean (LSMs) differences (gemigliptin vs. placebo) and P values were calculated. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
A total of 400 patients were screened, 315 patients were randomized, and 313 patients received gemigliptin (159 patients) or placebo (154 patients). Among these, 154 and 147 patients completed the MTP and started receiving gemigliptin 50 mg from week 24 in the gemigliptin and placebo groups, respectively. Of these, 151 patients (94.97%) in the gemigliptin group and 145 patients (92.95%) in the placebo group completed ETP up to week 52 (Fig. 1).
Baseline characteristics were comparable between the gemigliptin and placebo groups, except for BMI (25.96 kg/m2 vs. 27.13 kg/m2, P=0.0062). The mean age of the patients was 55.66 and 54.98 years in the gemigliptin and placebo groups, respectively. The mean HbA1c levels at baseline were 7.83% and 7.86% in the gemigliptin and placebo groups, respectively. The baseline results of renal function tests, including alanine aminotransferase, aspartate aminotransferase, and albuminuria (urine albumin-creatinine ratio [UACR]) were similar between the two groups (Table 1).
When added to metformin and dapagliflozin, gemigliptin significantly reduced HbA1c levels at week 24. The LSMs (standard error [SE]) of HbA1c changes from baseline at week 24 were –0.86% (0.05) and –0.20% (0.05) in the gemigliptin and placebo groups, respectively. The LSM difference (SE) in the HbA1c changes between the two groups was –0.66% (0.07) with a 95% CI of –0.80% to –0.52%, indicating superior glycemic control in the gemigliptin group compared to the placebo group (Table 2).
In addition to the HbA1c change at week 24, the HbA1c and FPG levels in the gemigliptin group were significantly reduced compared with those in the placebo group at each point of efficacy evaluation during the MTP for 24 weeks (P<0.0001) (Fig. 2).
The proportion of responders who achieved HbA1c levels <7% or <6.5% was significantly greater in the gemigliptin group than in the placebo group at week 24 (P<0.0001) (Fig. 3A, B). At week 52, the proportion of responders who achieved HbA1c <7% or <6.5% significantly increased in the placebo group, whereas the proportion of responders in the gemigliptin group was similar to that in the gemigliptin group observed at week 24 (Fig. 3C, D).
At week 24, total cholesterol and low density lipoprotein cholesterol significantly decreased from the baseline in the gemigliptin group compared to the placebo group and no significant change in high density lipoprotein cholesterol and triglyceride was observed for both groups (data not shown).
To evaluate renal function, eGFR and albuminuria (UACR) were measured. The eGFR increased from the baseline in both groups. UACR increased in the placebo group but decreased in the gemigliptin group at week 24 (Supplemental Table S2). However, the between-group differences were not statistically significant for either eGFR or UACR.
As the patients in the placebo group switched to gemigliptin 50 mg from week 24, both HbA1c and FPG levels were significantly reduced from baseline at each time point during the ETP (P<0.0001). As the patients in the gemigliptin group continued to receive gemigliptin 50 mg up to week 52, the efficacy on HbA1c and FPG reduction observed during the MTP was maintained up to week 52 in the gemigliptin group, considering that the LSM of HbA1c changes from baseline at week 24 was –0.86% and those at weeks 32, 40, and 52 ranged from –0.84% to –0.77% (Fig. 2).
During the OTP, only a few patients received rescue therapy (1.27% [2/158] in the gemigliptin group and 3.90% [6/154] in the placebo group). During the OTP, body weight fluctuated, but there was no significant change in body weight from baseline for both the gemigliptin (–0.01 kg) and placebo groups (–0.46 kg) (Supplemental Fig. S2A) and decrease in waist circumference was observed in both groups at each point during the OTP (Supplemental Fig. S2B).
During the MTP, 44 patients (27.67%) and 45 patients (29.22%) in the gemigliptin and placebo groups, respectively, reported treatment-emergent adverse events (TEAEs). Three patients in each group experienced serious adverse events (SAEs). None of the SAEs were reported to have a causal relationship. Urinary tract infection in three patients (1.89%) and one patient (0.65%) in the gemigliptin and placebo groups, respectively and genital infection in one patient each group was reported. All events of urinary tract infection and genital infection were considered unrelated to the study drug.
During the OTP, 60 patients (37.74%) and 66 patients (42.86%) reported TEAEs in the gemigliptin and placebo groups, respectively, with no noticeable increase in the incidence of TEAEs in the placebo group despite the switch to gemigliptin. Hypoglycemia was reported in two patients (1.26%, three events) in the gemigliptin group (gemigliptin 50 mg) during the OTP (Table 3). Two events were equivalent to level 1 (blood glucose level ≤70 mg/dL) and one event was equivalent to level 2 (blood glucose level <54 mg/dL); all were considered unrelated to the study drug [23,24]. All three patients recovered without the need for additional treatment.
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study conducted in T2DM patients who had inadequate glycemic control with metformin and dapagliflozin, the HbA1c reduction from baseline was significantly greater in the gemigliptin group at week 24 than in the placebo group. The mean HbA1c change (–0.86%) in the gemigliptin group was similar to the mean HbA1c changes (–0.87% to –0.68%) in previous clinical studies on the add-on therapy of gemigliptin to metformin with or without sulfonylurea and insulin with or without metformin [17,19,21]. Moreover, the therapeutic efficacy of gemigliptin compared to the placebo group (–0.66%) in this study was found to be greater than that (–0.47% to –0.35%) in the similar clinical studies in which a DPP-4 inhibitor was added on to an SGLT2 inhibitor and metformin [13,15]. Considering that there was statistically significant difference in baseline BMI between the two groups despite the small numerical difference (BMI 25.96 kg/m2 vs. 27.13 kg/m2), the post hoc analyses for the primary endpoint considering baseline BMI as additional fixed effect were performed. The LSM difference (SE) in the HbA1c changes between the two groups was –0.65% (0.07; with a 95% CI of –0.79% to –0.51%), indicating reaffirmed superior glycemic control in the gemigliptin group compared to the placebo group.
The glycemic control of gemigliptin was further supported by the secondary outcomes (HbA1c and FPG levels over time and the proportion of responders with HbA1c <7% or <6.5%) in the gemigliptin group. The LSMs of HbA1c change from baseline at weeks 6, 12, and 18 were –0.60%, –0.83%, and –0.86%, respectively, in the gemigliptin group, showing that HbA1c level tends to be stabilized at week 12, which is similar to the trend observed in similar clinical studies in which a DPP-4 inhibitor was added to an SGLT2 inhibitor and metformin [13,15].
As all patients in both the gemigliptin and placebo groups received gemigliptin 50 mg from week 24, HbA1c and FPG levels significantly decreased at each time point in the placebo group during the ETP, whereas the decreased HbA1c and FPG levels were maintained during the ETP in the gemigliptin group. The LSM of HbA1c changes from baseline at week 52 in the gemigliptin group (–0.77%) was found to be greater than the mean HbA1c change at week 52 from baseline (–0.38%) in a similar clinical study in which DPP-4 inhibitor was added to metformin and SGLT2 inhibitor [14].
At the end of the follow-up period, there was a slight increase in HbA1c from the observed nadirs for both groups and an increase in FPG from the observed nadir for the placebo group, which started receiving gemigliptin from week 24. Trends of small but progressive increases in HbA1c and FPG levels from the nadirs were also observed in similar long-term follow-up clinical studies with triple combination therapy [14,25-27]. Nonetheless, both HbA1c and FPG levels significantly decreased after gemigliptin treatment, and the efficacy of gemigliptin in glycemic control was stabilized and sustained for 52 weeks.
As DPP-4 inhibitors are known to be weight-neutral [24], no significant change in body weight was observed in patients receiving gemigliptin (–0.13 to 0.13 kg) compared to significant decrease in body weight observed in patients receiving the placebo (–0.64 to –0.32 kg) during the MTP for 24 weeks, despite the presence of dapagliflozin—with benefits of weight loss—as the background therapy for both groups, which is similar to the trend observed in similar studies adding DPP-4 inhibitors to existing therapies, including dapagliflozin [14,15]. The slightly higher mean baseline body weight in the placebo group and the reduction in glycosuria possibly caused by the powerful glucose-lowering effect of gemigliptin may have contributed to small change in body weight in patients receiving gemigliptin. These findings should be interpreted with caution considering that the maximum mean weight loss was less than 0.7 kg in the placebo group despite the statistical significance and no significant difference in waist circumference changes between the two groups was observed.
The incidence rates of TEAEs were similar between the gemigliptin and placebo groups during the MTP. The incidence rates of TEAEs in the placebo group during the ETP when the patients started receiving gemigliptin 50 mg did not differ from those during the MTP. Furthermore, no clinically significant difference compared with placebo was observed during the ETP. The incidence rates of TEAEs during the OTP were similar to those reported in clinical studies on saxagliptin added to dapagliflozin and metformin [14]. Moreover, the incidence rates of hypoglycemia were low and similar to those in previous clinical studies with gemigliptin as an add-on, suggesting that gemigliptin can be added to existing therapy without increasing the risk of hypoglycemia [19,21].
Genitourinary tract infections are the most common AE occurred during SGLT2 inhibitor-including therapy and the incidence of urinary tract infection and genital infection is 4.3% and 2.7%–6.9%, respectively observed in trials using dapagliflozin 10 mg containing therapy without DPP-4 inhibitor [28]. Besides the improved glucose-lowering effect of combination of DPP-4 inhibitor and SGLT2 inhibitor, addition of DDP-4 inhibitor to SGLT2 inhibitor may be beneficial in decrease in the risk of genitourinary tract infections, especially genital infection [29,30]. This study enrolled patients who had previously received SGLT2 inhibitor and metformin for glycemic control and it was expected that they would be relatively tolerable regarding urinary tract infection and genital infection. In fact, similarly low incidence rates of urinary tract infection (less than 2%) and genital infection (less than 1%) were reported for both groups. Considering the small number of incidences and unrelated causality of events, further studies are necessary to draw any conclusion. As this study was designed to evaluate the long-term efficacy and safety of gemigliptin following the guidelines of the European Medicines Agency (EMA) and the National Institute of Food and Drug Safety Evaluation [31,32], the long-term efficacy of gemigliptin on HbA1c reduction was confirmed, and the long-term safety of gemigliptin was evaluated up to 52 weeks.
In this study, the sustained long-term efficacy of gemigliptin, with no new clinically significant safety findings, was observed in T2DM patients when added to existing dapagliflozin and metformin therapy. Taken together, the add-on therapy with gemigliptin can be a potent solution for both the efficacy of glycemic reduction and safety, with low risks of hypoglycemia and weight gain in patients with T2DM who have inadequate glycemic control with dapagliflozin and metformin.
Supplementary Material
Supplemental Table S2.
Change from Baseline in eGFR and Albuminuria at Week 24–Full Analysis Set
Supplemental Table S3.
Contributing Investigators and Their Affiliations
Supplemental Fig. S1.
Study design. PO, per oral; qd, once a day. aPatients should be on medication (stable dose of dapagliflozin 10 mg/day+metformin ≥1,000 mg/day) for ≥8 weeks before screening; bPatients who had taken oral hypoglycemic agents in addition to dapagliflozin and metformin within 8 weeks before screening should stop this medication and undergo the washout period; cFor the patients who undergo the washout period, week –2 is the visit 1-1.
Supplemental Fig. S2.
(A) Change of body weight over time from baseline–full analysis set. (B) Change of waist circumference over time from baseline–full analysis set. LS, least square; SE, standard error. aP<0.05 vs. gemigliptin.
Notes
ACKNOWLEDGMENTS
The authors thank all the investigators (Supplemental Table S3), study site staff, and patients for their participation in this study (SOLUTION), as well as EunJin Seok of LG Chem, Ltd. for supporting the clinical operations, SeokHyun Oh of LG Chem, Ltd. for providing statistical support, and Sijin Park of LG Chem, Ltd. for writing assistance in preparing the manuscript.
REFERENCES
1. DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014; 27:100–12.

2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007; 298:194–206.

3. Holst JJ, Deacon CF. Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol. 2004; 4:589–96.

4. Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008; 4:753–68.
5. Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998; 47:1663–70.

6. Korean Diabetes Association. Clinical practice guidelines for diabetes. 7th ed. Seoul: Korean Diabetes Association;2021.
7. Shah NK, Deeb WE, Choksi R, Epstein BJ. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2012; 32:80–94.

8. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010; 375:2223–33.

9. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:323–34.

10. Heise T, Seewaldt-Becker E, Macha S, Hantel S, Pinnetti S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:613–21.

11. Dey J. SGLT2 inhibitor/DPP-4 inhibitor combination therapy: complementary mechanisms of action for management of type 2 diabetes mellitus. Postgrad Med. 2017; 129:409–20.
12. Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016; 12:1407–17.

13. Matthaei S, Catrinoiu D, Celinski A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015; 38:2018–24.

14. Matthaei S, Aggarwal N, Garcia-Hernandez P, Iqbal N, Chen H, Johnsson E, et al. One-year efficacy and safety of saxagliptin add-on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016; 18:1128–33.

15. Tinahones FJ, Gallwitz B, Nordaby M, Gotz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as add-on to empagliflozin and metformin in patients with type 2 diabetes: two 24-week randomized, double-blind, double-dummy, parallel-group trials. Diabetes Obes Metab. 2017; 19:266–74.

16. Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:410–6.

17. Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013; 15:523–30.

18. Lim S, Han KA, Yu J, Chamnan P, Kim ES, Yoon KH, et al. Efficacy and safety of initial combination therapy with gemigliptin and metformin compared with monotherapy with either drug in patients with type 2 diabetes: a double-blind randomized controlled trial (INICOM study). Diabetes Obes Metab. 2017; 19:87–97.

19. Ahn CH, Han KA, Yu JM, Nam JY, Ahn KJ, Oh TK, et al. Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study). Diabetes Obes Metab. 2017; 19:635–43.

20. Yoon SA, Han BG, Kim SG, Han SY, Jo YI, Jeong KH, et al. Efficacy, safety and albuminuria-reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: a 12-week, double-blind randomized study (the GUARD Study). Diabetes Obes Metab. 2017; 19:590–8.

21. Cho YM, Deerochanawong C, Seekaew S, Suraamornkul S, Benjachareonwong S, Sattanon S, et al. Efficacy and safety of gemigliptin as add-on therapy to insulin, with or without metformin, in patients with type 2 diabetes mellitus (ZEUS II study). Diabetes Obes Metab. 2020; 22:123–7.

22. Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009; 25:2401–11.
23. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017; 40:155–7.
24. American Diabetes Association. Standards of medical care in diabetes: 2022. Diabetes Care. 2022; 45(Suppl 1):S1–264.
25. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, Hansen L, Chen H, Johnsson E, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016; 18:1134–7.

26. Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab. 2011; 13:1088–96.

27. Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013; 36:2508–15.
28. AstraZeneca Pharmaceutical. Farxiga [Internet]. Silver Spring: U.S. Food and Drug Administration;2022. [cited 2023 May 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/202293s028lbl.pdf.
29. Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018; 20:740–4.

30. Li D, Shi W, Wang T, Tang H. SGLT2 inhibitor plus DPP-4 inhibitor as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018; 20:1972–6.

31. EMA Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet]. London: European Medicines Agency;2012. [cited 2023 May 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf.
32. National Institute of Food and Drug Safety Evaluation. Guideline on clinical trials of oral hypoglycemic agent [Internet]. Cheongju: Ministry of Food and Drug Safety;2015. [cited 2023 May 12]. Available from: https://www.mfds.go.kr/index.do.
Fig. 1.
Disposition. aSafety set includes all randomized patients administered with the investigational product (placebo or gemigliptin) at least once; bFull analysis set includes patients in the safety set with hemoglobin A1c level measured at baseline and at least once after

Fig. 2.
(A) Change of hemoglobin A1c (HbA1c) over time from baseline–full analysis set. (B) Change of fasting plasma glucose (FPG) over time from baseline–full analysis set. LS, least square; SE, standard error. aP<0.0001 vs. placebo.
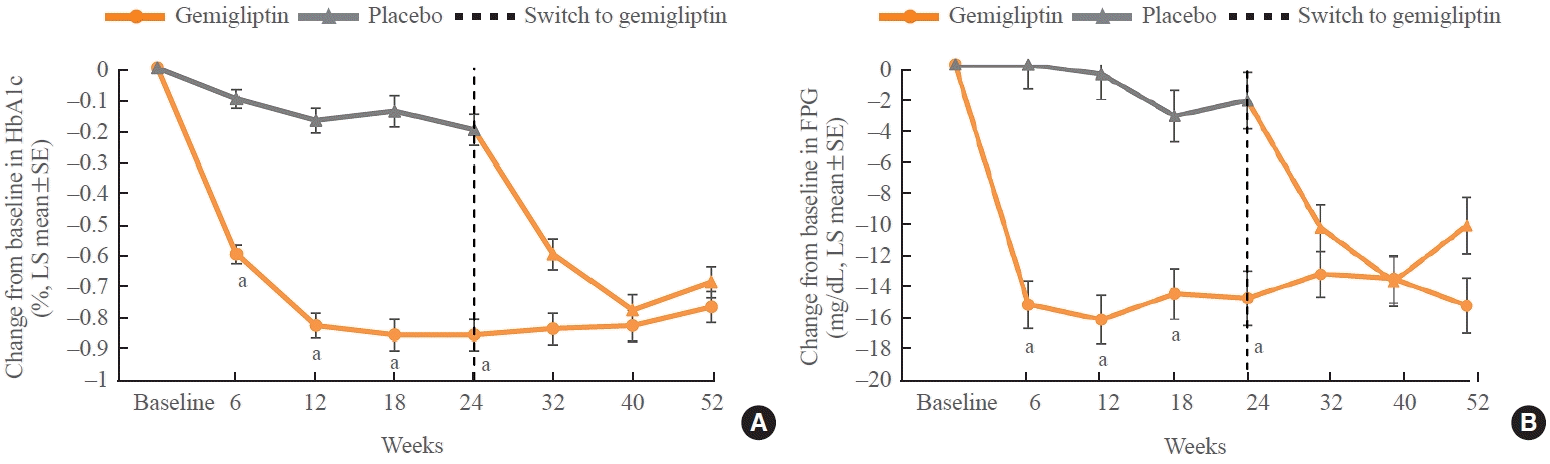
Fig. 3.
(A) Adjusted responder rate (hemoglobin A1c [HbA1c] <7%) at week 24–full analysis set. (B) Adjusted response rate (HbA1c <6.5%) at week 24–full analysis set. (C) Adjusted response rate (HbA1c <7%) at week 52–full analysis set. (D) Adjusted response rate (HbA1c <6.5%) at week 52–full analysis set. OR, odds ratio; CI, confidence interval. aLogistic regression model including group as a factor and HbA1c and estimated glomerular filtration rate values as covariates.
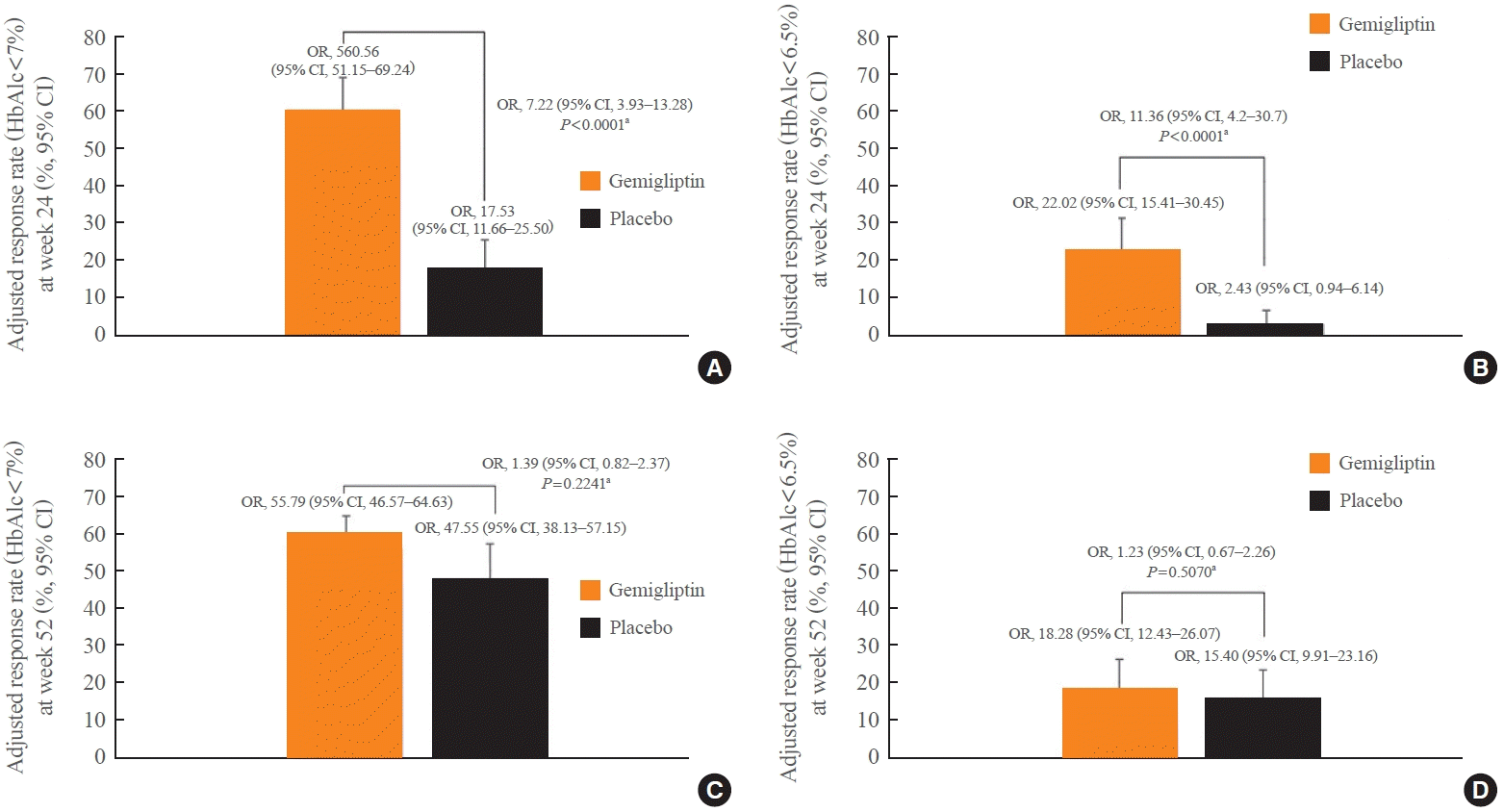
Table 1.
Demographics and Baseline Characteristics–Full Analysis Set
| Characteristic | Gemigliptin 50 mg (n=158) | Placebo (n=154) |
|---|---|---|
| Age, yr | 55.66±9.79 | 54.98±9.90 |
| Male sex | 99 (62.66) | 81 (52.60) |
| Body weight, kg | 71.06±12.52 | 73.64±12.65 |
| Body mass index, kg/m2a | 25.96±3.29 | 27.13±3.77b |
| Duration of diabetes, yr | 8.83±5.83 | 8.24±4.99 |
| Taking additional oral hypoglycemic agents | ||
| Yes | 1 (0.63) | 5 (3.25) |
| No | 157 (99.37) | 149 (96.75) |
| HbA1c, % | 7.83±0.68 | 7.86±0.77 |
| FPG, mg/dL | 139.80±23.99 | 136.62±23.48 |
| eGFR, mL/min/1.73 m2 | 92.69±16.85 | 92.51±19.32 |
| Metformin dose, mg/day | 1453.16±444.11 | 1415.58±432.40 |
| Albuminuria, mg/g | 45.65±122.44 | 41.84±77.70 |
| ALT, IU/L | 26.71±14.29 | 27.46±16.63 |
| AST, IU/L | 23.76±9.28 | 24.98±14.82 |
Table 2.
Change in HbA1c from Baseline at Week 24–Full Analysis Set
| Variable | Gemigliptin 50 mg (n=158) | Placebo (n=154) |
|---|---|---|
| Baseline, mean±SD | 7.83±0.68 | 7.86±0.77 |
| Week 24, mean±SD | 6.98±0.75 | 7.60±0.82 |
| Change from baseline at week 24 | ||
| LSM (SE) | –0.86 (0.05) | –0.20 (0.05) |
| 95% CI for LSM | –0.96 to –0.76 | –0.30 to –0.10 |
| LSM difference (SE) | –0.66 (0.07) | |
| 95% CI for LSM difference | –0.80 to –0.52 | |
| P valuea | <0.0001 | |
Table 3.
Safety Results–Safety Set
| TEAEa |
Main treatment period |
Overall treatment periodb |
|||
|---|---|---|---|---|---|
| Gemigliptin 50 mg (n=159) | Placebo (n=154) | Gemigliptin 50 mg (n=159) | Placebo (n=154) | ||
| Any TEAEs | 44 (27.67) | 45 (29.22) | 60 (37.74) | 66 (42.86) | |
| Hypoglycemia | 1 (0.63) | 0 | 2 (1.26) | 0 | |
| Arthralgia | 0 | 1 (0.65) | 0 | 2 (1.30) | |
| Pemphigoid | 0 | 0 | 0 | 0 | |
| Hypersensitivity | 0 | 0 | 0 | 0 | |
| Pancreatitis acute | 0 | 0 | 0 | 0 | |
| AST increased | 0 | 2 (1.30) | 0 | 3 (1.95) | |
| ALT increased | 0 | 2 (1.30) | 0 | 3 (1.95) | |
| Urinary tract infectionc | 3 (1.89) | 1 (0.65) | 3 (1.89) | 2 (1.30) | |
| Genital infectiond | 1 (0.63) | 1 (0.65) | 3 (1.89) | 2 (1.30) | |
| Any TEAE related to study drug | 2 (1.26) | 1 (0.65) | 2 (1.26) | 1 (0.65) | |
| Any TESAEs | 3 (1.89) | 3 (1.95) | 3 (1.89) | 7 (4.55) | |
| Any TESAE related to study drug | 0 | 0 | 0 | 0 | |
| Any TEAEs leading to discontinuation | 1 (0.63) | 2 (1.30) | 2 (1.26) | 2 (1.30) | |
Values are expressed as number (%).
TEAE, treatment-emergent adverse event; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TESAE, treatment-emergent serious adverse event.
a Treatment-emergent adverse events were coded using the Medical Dictionary for Regulatory Activities version 24.0;




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download