Abstract
Dihydroaustrasulfone alcohol (DA), the synthetic precursor of a natural compound (austrasulfone) isolated from the coral species Cladiella australis, has shown cytotoxic effects against cancer cells. However, it is unknown whether DA has antitumor effects on nasopharyngeal carcinoma (NPC). In this study, we determined the antitumor effects of DA and investigated its mechanism of action on human NPC cells. The MTT assay was used to determine the cytotoxic effect of DA. Subsequently, apoptosis and reactive oxygen species (ROS) analyses were performed by using flow cytometry. Apoptotic and PI3K/AKT pathway-related protein expression was determined using Western blotting. We found that DA significantly reduced the viability of NPC-39 cells and determined that apoptosis was involved in DA-induced cell death. The activity of caspase-9, caspase-8, caspase-3, and PARP induced by DA suggested caspase-mediated apoptosis in DA-treated NPC-39 cells. Apoptosis-associated proteins (DR4, DR5, FAS) in extrinsic pathways were also elevated by DA. The enhanced expression of proapoptotic Bax and decreased expression of antiapoptotic BCL-2 suggested that DA mediated mitochondrial apoptosis. DA reduced the expression of pPI3K and p-AKT in NPC-39 cells. DA also reduced apoptosis after introducing an active AKT cDNA, indicating that DA could block the PI3K/AKT pathway from being activated. DA increased intracellular ROS, but N-acetylcysteine (NAC), a ROS scavenger, reduced DA-induced cytotoxicity. NAC also reversed the chances in pPI3K/AKT expression and reduced DA-induced apoptosis. These findings suggest that ROSmediates DA-induced apoptosis and PI3K/AKT signaling inactivation in human NPC cells.
Nasopharyngeal carcinoma (NPC) is a distinctive type of head and neck cancer due to its geographic distribution. NPC affects an estimated 130,000 patients worldwide, accounting for 0.7% of all cancer cases in 2018. The highest rates are in Southern China, Southeast Asia, and North Africa, where NPC is consistently associated with Epstein‒Barr virus infection [1].
Radiotherapy alone is adequate for the early stages of NPC, but radiation combined with platinum-based chemotherapy is the mainstay treatment for locally advanced NPC [2-5]. Distant metastasis is the predominant cause of treatment failure and accounts for cancer-specific mortality among approximately 70% of cancer patients [6,7]. Current chemotherapy agents used to treat advanced NPC have limitations regarding therapeutic potential and the duration of response. Malignant tumors eventually develop resistance. The search for the most effective drugs and optimal treatment regimens remains a significant challenge.
Oceans cover more than 70% of the Earth’s surface. The diversity of marine flora and fauna is an untapped source of medical discovery. Previous studies have reported the broad-spectrum biological effects of natural compounds isolated from Taiwanese soft corals. For example, capnellene and lemnalol have anti-inflammatory and neuroprotective properties [8,9]. Cembrane-type diterpenes, such as sinularin and 5-epi-sinuleptolide, also show anticancer activity [10-12].
Dihydroaustrasulfone alcohol (DA) is the synthetic bioactive precursor of austrasulfone, which is isolated from the Formosan (Taiwanese) soft coral Cladiella australis [13]. Previous studies have shown the therapeutic properties of DA, including anti-inflammatory and neuroprotective properties, the attenuation of neuropathic pain, the ability to treat multiple sclerosis and non-small cell lung cancer [13,14], the alleviation of atopic dermatitis [15], and inhibiting angiogenesis in atherosclerosis [16]. However, it is unknown whether DA has antitumor effects on NPC. Previously, researchers found that the PI3K and MAPK pathways play critical roles in the anticancer effect of DA on A549 cells [13]. In addition, we further investigated the apoptosis-related and reactive oxygen species (ROS)-dependent mechanisms underlying the therapeutic effects of DA while evaluating its anticancer activity against NPC and the PI3K pathway in this study.
The NPC-39 and NPC-BM cell lines were used in this study, which are NPC cells and were obtained from Dr. Mu-Kuan Chen at Changhua Christian Hospital (Taiwan). Both cell lines were cultured in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin‒streptomycin (Biological Industries) and maintained in a 5% CO2 and 37°C incubator.
DA was synthesized as reported previously [13]. In brief, the initial I,4-addition of 2-mercaptoethanol to methyl vinyl ketone to generate the corresponding sulfide compound and the subsequent oxidation of this sulfide with m-chloroperoxybenzoic acid were performed. Nuclear magnetic resonance spectroscopy was used to characterize the product’s structure (Supplementary Fig. 1), and the sample was stored at −20°C following dissolution in dimethyl sulfoxide (DMSO).
Cells in culture plates were treated with DA for 24 h, followed by an MTT assay. MTT solution (5 mg/ml; Merck Millipore) was directly added to the culture wells and incubated for 4 h. The formazan was dissolved in 300 µl of DMSO/well, and the absorbance was measured at 540 nm with an ELISA device.
The protocol of the colony-formation assay in this study was adapted from previous reports with necessary modifications [17]. NPC cells in six-well plates were incubated for 24 h, followed by incubation with DA-containing complete medium for seven days to visualize the colonies. The colonies were fixed with acetic acid and methanol (1:3) and stained with 2% crystal violet before being counted under an inverted microscope (Olympus).
NPC cells were treated with DA-containing medium for at least 24 h before being harvested for staining with propidium iodide (PI) (50 µg/ml) and Annexin V-FITC (20 µg/ml) (Biolegend) at room temperature for 15 min. Apoptotic cells were quantified by an Accuri C5 flow cytometer (BD Biosciences).
CaspGLOW fluorescein active caspase staining kits (Biovision) were used to measure caspase activities, including caspase-3, -8, and -9. Cells were treated with DA-containing medium for 24 h, harvested, centrifuged at 1,200 rpm, washed with phosphate-buffered saline (PBS), and finally incubated with a caspase staining kit according to the manufacturer’s instructions before being analyzed by flow cytometry. In addition, Z-DEVD-FMK, an inhibitor of caspase-3, was used to treat the cells 2 h before DA treatment to rescue NPC cell viability, which was measured by MTT assays. For cell cycle analysis, intracellular PI staining was used to determine the DNA content linked to the cell cycle status.
DA-treated cells were collected and lysed with ice-cold RIPA buffer (MedChemExpress), and a phosphatase inhibitor (Sigma-Aldrich) was added to prevent the degradation of phosphorylated proteins. Forty micrograms of total protein was subsequently separated by SDS‒PAGE and hybridized with the following antibodies after being transferred to PVDF membranes (Merck Millipore) and blocked with blocking buffer (Visual Protein). The primary antibodies were diluted 1:100 and included anti-GAPDH (Abcam, cat. no. ab9484), anti-Bax (Biolegend, cat. no. 633602), anti-Bcl-2 (Cell Signaling, cat. no. 2870s), anti-cleaved-PARP (Cell Signaling, cat. no. 9541s), anti-pPI3K (Cell Signaling, cat. no. 13857s), anti-PI3K (Santa Cruz, cat. no. sc-136208), anti-AKT (Sigma-Aldrich, cat. no. 05-1003), and anti-pAKT (Santa Cruz, cat. no. sc-7985); then, a 1:2,000 dilution of horseradish peroxidase-conjugated secondary antibodies (cat. no. 111-035-003, Jackson ImmunoResearch Laboratories, Inc.) was added and incubated with enhanced chemiluminescence (GE Healthcare Life Sciences) in a Hansor Luminescence Image system (Hansor).
DA-treated NPC cells were analyzed with a JC-1 Assay Kit (Invitrogen Life Technologies) and FITC-anti-cytochrome c antibodies to determine mitochondrial membrane potential and cytochrome c release, respectively. Briefly, the cells were incubated with medium containing JC-1 fluorescent dye or cytochrome c solution, including the specific antibody, for 10 min in the dark at 37°C prior to analysis with an Accuri C5 flow cytometer. The results were analyzed using BD Accuri C6 Software version 1.0.264.21.
DA-treated cells were trypsinized and stained with anti-DR4-FITC, anti-DR5-FITC, and anti-Fas-FITC (eBioscience) antibodies at 4°C for 30 min. The expression was detected using an Accuri C5 flow cytometer and analyzed using BD Accuri C6 Software version 1.0.264.21.
The pBabe.puro-Myc-Flag-PKBA/AKT plasmid was obtained from Addgene (Watertown) and transfected into HEK-293T cells with jetPEM transfection reagent for 24 h to generate viral particles, including gag-pol and VSV-G proteins, as instructed by the manufacturer. The viral particles were collected to infect NPC-39 cells (5 × 105/well) for 48 h, and the harvested cells were plated in culture plates for further experiments.
Intracellular ROS concentrations were determined using the peroxide-sensitive fluorescent probe DCFH–DA. In brief, DA-treated NPC cells were collected, washed twice with PBS, and incubated with 1 µl of DCFDA in 1 ml of HBSS buffer (Invitrogen) for 30 min in an incubator. After further washing with PBS, the level of ROS was determined by flow cytometry and the corresponding software. N-acetylcysteine (NAC, Sigma-Aldrich)-treated cells were used as a positive control to compare the ROS scavenging activity of DA, and MTT assays were used to determine cell viability.
Unless indicated elsewhere, all data are presented as the mean ± standard deviation. Data in groups were further analyzed for significant differences by one-way ANOVA followed by Tukey’s posttest or unpaired two-tailed t-test. A p-value less than 0.05 was considered to be statistically significant.
As shown in Fig. 1B, treatment of NPC-39 and NPC-BM cells with DA (Fig. 1A) (0−100 µM) for at least 24 h reduced their proliferation in a dose-dependent manner. The MTT assay showed that the IC50 values of DA were 27.1 ± 4.6 and 45.4 ± 7.3 µM in NPC-39 and NPC-BM cells, respectively. In parallel, colony formation assays demonstrated that DA treatment for one week inhibited NPC-39 and NPC-BM cells from forming more colonies compared to control cells (Fig. 1C, D). These results suggest that DA could reduce the growth of NPC cells. We observed that NPC-39 cells appeared more susceptible to DA treatment than NPC-BM cells. As a result, we assume that the NPC-39 cell line is more adequate for the following experiments to better illustrate the anticancer effects of DA in vitro.
To determine whether the death of NPC cells induced by DA resulted from apoptosis, we stained cells with Annexin V conjugated FITC and PI to label DNA contents, followed by flow cytometry to analyze the degrees of apoptosis and cell-cycle distribution with or without DA treatment for 24 h. The results in Fig. 2A and 2C illustrate that dead cells, representing the sub-G1 population, were significantly increased following DA treatment. Annexin V-positive cells were also increased by DA, indicating higher apoptotic ratios of NPC-39 cells (Fig. 2B, D). These results were further confirmed by determining poly (ADP-ribose) polymerase (PARP) protein expression levels via Western blot analysis, and we observed higher expression of PARP in NPC-39 cells, suggesting that the apoptotic protein marker was activated and elevated after DA treatment in a dose-dependent manner (Fig. 2E, F).
Furthermore, we attempted to explore the apoptotic pathways involved in the effect of DA treatment on NPC-39 cells. We directly measured the activities of caspase-9, caspase-8, and caspase-3 and found that DA dose-dependently increased the activities of all three caspases, including caspase-9 (Fig. 3A, B), caspase-8 (Fig. 3A, C), and caspase-3 (Fig. 3A, D), in NPC-39 cells. Consistently, we added the pancaspase inhibitor Z-VAD-FMK to DA-induced cells to determine whether the inhibitor could reverse apoptosis levels. The MTT assays demonstrate that Z-VAD-FMK significantly rescued the viability of NPC-39 cells after DA treatment (Fig. 3E), proving that the growth of NPC cells was slowed and that caspase-mediated apoptosis was promoted.
Mitochondrial dysfunction is generally associated with apoptosis. We stained cells with JC-1 dye to measure the mitochondrial membrane potential to understand whether DA-induced apoptosis involves mitochondrial dysfunction. The results showed that mitochondria in cells without DA treatment retained a high level of matrix metalloproteinase (MMP)-dependent JC-1, and this JC-1 aggregation was dose-dependently decreased by DA treatment (Fig. 4A, C). Moreover, the results in Fig. 4B and 4D show that cytochrome c release into the cytosol in DA-treated mitochondria was increased to a higher level than that in mitochondria without DA treatment. In addition, the protein expression levels of Bax were increased, and the antiapoptotic protein Bcl-2 was decreased following DA treatment (Fig. 5A, B), which was consistent with the increase in truncated BH3 interacting-domain death agonist (tBid) protein levels, indicating that mitochondrial dysfunction was involved in DA-induced carcinoma cytotoxicity (Fig. 5).
Given our previous data suggesting that caspase-8 activity was activated by DA (Fig. 3A, C) and the increase in truncated Bid (Fig. 5), both of which are associated with the extrinsic apoptotic pathway and are highly correlated with death receptors such as DR4, DR5, and Fas/CD95 (death receptor protein), we attempted to confirm the expression of the aforementioned death receptor proteins with and without DA treatment. We stained the indicated cell surface markers and analyzed the numbers of positive cells. The results revealed that the surface levels of DR4 (Fig. 6A, B), DR5 (Fig. 6A, C), and Fas (Fig. 6A, D) protein on the surface of NPC-39 cells increased in a concentration-dependent manner after DA treatment.
We wondered if the survival signaling pathway in NPC cells was interfered with and inhibited by DA, and so we examined the phosphorylation levels of the PI3K protein and its downstream protein AKT to examine the mechanisms of DA. The results in Fig. 7A and 7B show that phosphorylated PI3K and AKT were profoundly and dose-dependently decreased by DA, while the total protein levels of PI3K and AKT remained constant, suggesting that DA could inhibit the PI3K/AKT pathway in NPC-39 cells, leading to the cells receiving fewer survival signals. Therefore, we transfected NPC-39 cells to create cells constitutively expressing activated PI3K/AKT (Fig. 7C) to validate our findings. We found that overactivation of the AKT pathway overcame the adverse and cytotoxic effects of DA treatment, as evidenced by fewer Annexin V-positive cells (Fig. 7D, E) and rescued cell viability compared with cells treated with DA alone (Fig. 7F). These findings strongly suggest that the PI3K/AKT pathway is involved in DA-mediated induction of apoptosis or cytotoxicity in NPC-39 cells.
Because ROS can affect cellular redox status and mitochondrial membrane potential, we needed to confirm whether DA could promote ROS production in NPC-39 cells. As shown in Fig. 8, the DCFDA fluorescence intensity of DA-treated cells was significantly and dose-dependently increased compared to untreated cells. In addition, pretreatment of NPC-39 cells with the oxidant scavenger NAC rescued cell viability, and DA-treated cells without NAC exhibited higher cytotoxicity (Fig. 9A). Furthermore, a decrease in apoptosis was also observed in NAC-treated cells by flow cytometric analysis, suggesting that ROS were a key factor in the therapeutic effect of DA (Fig. 9B, C). Notably, NAC treatment significantly reversed phospho-AKT expression (Fig. 9D), suggesting that DA could inhibit cell growth and inactivate the phosphorylation of PI3K/AKT signaling, both of which were associated with ROS levels induced by DA.
The ocean accounts for over two-thirds of the Earth’s surface and provides valuable natural resources with biodiversity. Many marine compounds derived from organisms have been shown to have anticancer effects, demonstrating their potential for cancer treatment [18-22]. DA, which is derived from coral Cladiella australis, has been shown to exert cytotoxic effects against cancer cells. Chen et al. [14] demonstrated that DA inhibited the migration of A549 lung cancer cells and MMP-2/-9 expression. The researchers also found that this compound attenuated the phosphorylation of PI3K, AKT, p38, ERK1/2, and c-Jun N-terminal kinase 1/2, as well as PI3K expression [14]. However, the antitumor effect and detailed mechanism of DA in human NPC cells are unknown. We examined the proliferation of DA-treated NPC cells in vitro, and the results indicated a significant reduction in the viability of NPC-39 and NPC-BM cells. We found that NPC-39 cells were more sensitive to DA treatment and therefore more suitable for investigating anticancer effects in vitro.
Apoptosis is a mode of programmed cell death promoted by anticancer treatments. Our data demonstrated that DA induced NPC-39 apoptosis, which was confirmed by flow cytometric gating with Annexin V-FITC, PI staining, and examination of a sub-G1 population. Caspases are protease enzymes that play an essential role in apoptosis. The caspase cascade is an intrinsic or an extrinsic apoptosis pathway.
Members of the TNF superfamily, including TNF, Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL), initiate the extrinsic apoptosis pathway. These ligands induce apoptosis by binding to their respective receptors: Fas (CD95), TNFR1, TRAIL-R1 (DR4), and TRAIL-R2 (DR5). Trimerization of the receptor and clustering of the receptor’s intracellular death domain (DD) leads to the recruitment of the adaptor molecule Fas-associated death domain or TNF receptor-associated protein with death domain, which forms a death-inducing signaling complex (DISC) and subsequently activates caspase-8 and -10. Activated caspase-8 and -10 then cleave caspase-3, which leads to the cleavage of death substrates. DISC-mediated caspase activation is regulated by the cellular FLICE-like inhibitory protein [23-25].
The intrinsic pathway, which is also known as the mitochondrial pathway, involves activating the proapoptotic arm of the Bcl-2 gene superfamily. Mitochondrial outer membrane permeabilization and the release of cytochrome c into the cytosol occur via the intrinsic route. Cytochrome c interacts with the adaptor APAF-1 in the cytosol, producing an apoptosome that activates caspase-9, an apoptosis-initiating protease. Other executioner caspases are cleaved and activated by Caspase-9 (caspase-3, caspase-6, and caspase-7) [26-29].
Our study showed increased caspase-9, caspase-8, caspase-3, and PARP activity in DA-treated NPC-39 cells. In addition, a pancaspase inhibitor (Z-VAD-FMK) was proven to have a protective effect. These results verified that caspase-mediated apoptosis was involved in the treatment process. Increased expression of DR4, DR5, and FAS was also identified in the DA-treated cells, indicating that DA is involved in the extrinsic apoptosis pathway. Furthermore, decreased JC-1 and MMP aggregation and increased cytochrome c levels suggested mitochondrial apoptosis driven by DA.
Intrinsic and extrinsic apoptosis signaling pathways communicate with one another. The proapoptotic Bcl-2 family member Bid is activated by caspase-8-mediated cleavage. The interaction of Bax and Bak with caspase-8 leads to the separation of Bid and translocation of tBid to mitochondria, resulting in increased cytochrome c release, providing a plausible molecular link between the extrinsic and intrinsic pathways [28].
Bcl-2 (B-cell lymphoma 2), which is encoded by the BCL2 gene, is a critical protein in the Bcl-2 family that regulates cell death. Bcl-2 drives this process by inhibiting or inducing apoptosis. The proapoptotic proteins in the BCL-2 family, including Bax and Bak, promote mitochondrial outer membrane permeability and the release of cytochrome c and ROS, which are essential signals in the apoptosis cascade. These proapoptotic proteins are activated by BH3-only proteins but inhibited by antiapoptotic BCL-2 and its relative BCL-XL [27,30,31]. This study demonstrated increased proapoptotic Bax expression and decreased antiapoptotic BCL-2 expression in DA-treated NPC-39 cells. The findings suggest that DA promotes apoptosis in the balance between proapoptotic and antiapoptotic effects.
The PI3K/AKT signaling pathway is considered the “survival pathway” because of its essential role in cell differentiation, proliferation, and survival. AKT is upregulated in various malignant tumors, including gastric, colon, breast, ovarian, and prostate cancers [32,33]. The PI3K/AKT pathway has become an important target in cancer therapy. We demonstrated the inhibitory effects of DA on the expression of pPI3K and pAKT in NPC-39 cells, suggesting that DA could block the activation of the PI3K/AKT pathway. Moreover, we transfected DA-treated NPC-39 cells with an active form of AKT cDNA, and showed reduced apoptosis and the return of cell viability. This event aided the inactivation of the PI3K/AKT pathway by DA.
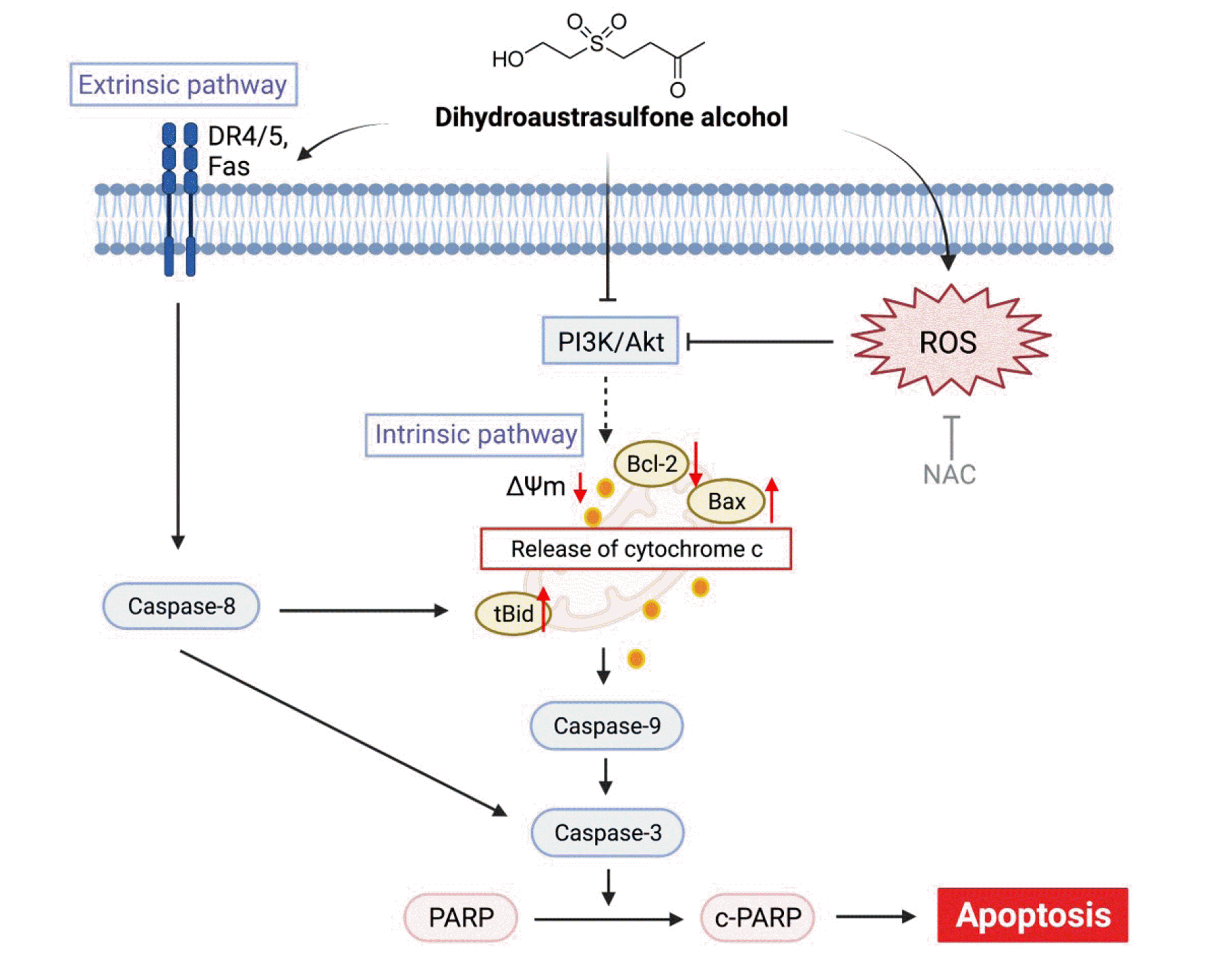
While we did not investigate the autophagy pathway, it is worth noting that an increase in ROS can downregulate the autophagic pathway, particularly ULK1 signaling, and the phosphorylation of PI3K mediated by ULK1 was also suppressed in a previous study [34]. In line with previous findings, we observed that the downregulation of PI3K and activation of apoptotic signaling by DA were highly dependent on ROS production. In summary, a model of the mechanism by which DA inhibits the growth of NPC could be proposed: DA treatment induces ROS production, which mitigates autophagy and activates apoptosis in carcinoma cells, eventually leading to the downregulation of PI3K.
Because ROS play a vital role in the survival of cancer cells, we explored ROS production in DA-treated NPC-39 cells. Cancer cells have higher ROS levels than normal cells because of their enhanced metabolism and persistent pro-oxidative state, which leads to intrinsic oxidative stress. Increased ROS levels are closely related to cancer initiation, metastasis, and drug resistance. However, elevated ROS also increase the susceptibility of tumor cells to oxidative stress and trigger cell death, which could be a potential strategy for cancer therapy [35,36]. Mitochondria are a possible ROS generation site that can leak single electrons to oxygen, convert them into superoxide anions and reduce mitochondrial transmembrane potential (ΔΨm). The mitochondrial apoptosis pathway is then triggered. In our study, ROS production was promoted in DA-treated NPC-39 cells, and the oxidant scavenger NAC was found to reduce DA-induced cell viability. This evidence suggests ROS-mediated cell death in DA-treated NPC-39 cells.
Previous studies have shown that ROS can repress the PI3K/AKT pathway [37-42]. To determine the relationship between PI3K/AKT activation and ROS production in response to DA treatment, we used the ROS scavenger NAC to investigate the activation of PI3K/Akt in DA-treated NPC-39 cells. We found that NAC significantly reduced DA-induced apoptosis and reversed pPI3K/AKT expression. These results indicated that ROS mediated DA-induced apoptosis and the inactivation of PI3K/AKT signaling in NPC-39 cells.
We demonstrated that DA inhibits the growth and proliferation of NPC-39 cells by inducing G1 phase cell cycle arrest. In addition, DA induces intrinsic and extrinsic pathways involving caspase-sdependent apoptosis in NPC-39 cells through ROS accumulation and the repression of pPI3K and pAKT. We examined ROS-mediated inactivation of the PI3K/AKT pathway in apoptosis in DA-treated NPC-39 cells. Fig. 10 shows the proposed mechanism of DA-induced apoptosis in NPC-39 cells. This is the first report showing the inhibitory effect of DA on cell proliferation and the induction of apoptosis in NPC cells in vitro. Our findings suggest that DA has therapeutic potential for treating NPC.
Supplementary data including one figure can be found with this article online at https://doi.org/10.4196/kjpp.2023.27.4.383.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Erratum in: CA Cancer J Clin. 2020;70:313. DOI: 10.3322/caac.21492. PMID: 30207593.

2. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, Hui EP, Yiu HY, Yeo W, Cheung FY, Yu KH, Chiu KW, Chan DT, Mok TS, Yau S, Yuen KT, Mo FK, Lai MM, Ma BB, Kam MK, et al. 2005; Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 97:536–539. DOI: 10.1093/jnci/dji084. PMID: 15812080.

3. Lee AWM, Tung SY, Ng WT, Lee V, Ngan RKC, Choi HCW, Chan LLK, Siu LL, Ng AWY, Leung TW, Yiu HHY, O'Sullivan B, Chappell R. 2017; A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer. 123:4147–4157. DOI: 10.1002/cncr.30850. PMID: 28662313.

4. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. 2003; Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 21:631–637. DOI: 10.1200/JCO.2003.06.158. PMID: 12586799.

5. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, Chua ET, Yang E, Lee KM, Fong KW, Tan HS, Lee KS, Loong S, Sethi V, Chua EJ, Machin D. 2005; Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 23:6730–6738. DOI: 10.1200/JCO.2005.16.790. PMID: 16170180.

6. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. 2011; How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 80:661–668. DOI: 10.1016/j.ijrobp.2010.03.024. PMID: 20643517.

7. Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, Chan OS, Chang AT, Yeung RM. 2014; Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 110:377–384. DOI: 10.1016/j.radonc.2014.02.003. PMID: 24630534.

8. Jean YH, Chen WF, Duh CY, Huang SY, Hsu CH, Lin CS, Sung CS, Chen IM, Wen ZH. 2008; Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur J Pharmacol. 578:323–331. DOI: 10.1016/j.ejphar.2007.08.048. PMID: 17916350.

9. Jean YH, Chen WF, Sung CS, Duh CY, Huang SY, Lin CS, Tai MH, Tzeng SF, Wen ZH. 2009; Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br J Pharmacol. 158:713–725. DOI: 10.1111/j.1476-5381.2009.00323.x. PMID: 19663884. PMCID: PMC2765592.

10. Huang HW, Tang JY, Ou-Yang F, Wang HR, Guan PY, Huang CY, Chen CY, Hou MF, Sheu JH, Chang HW. 2018; Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules. 23:849. Erratum in: Molecules. 2018;23:1670. DOI: 10.3390/molecules23071670. PMID: 29987258. PMCID: PMC6099670. PMID: 369b8cb3979044019e84e63bbde2a8ef.

11. Liang CH, Wang GH, Chou TH, Wang SH, Lin RJ, Chan LP, So EC, Sheu JH. 2012; 5-epi-Sinuleptolide induces cell cycle arrest and apoptosis through tumor necrosis factor/mitochondria-mediated caspase signaling pathway in human skin cancer cells. Biochim Biophys Acta. 1820:1149–1157. DOI: 10.1016/j.bbagen.2012.02.003. PMID: 22348919.

12. Wang SC, Li RN, Lin LC, Tang JY, Su JH, Sheu JH, Chang HW. 2021; Comparison of antioxidant and anticancer properties of soft coral-derived sinularin and dihydrosinularin. Molecules. 26:3853. DOI: 10.3390/molecules26133853. PMID: 34202721. PMCID: PMC8270243. PMID: b3e838ba6c3a41f2826da3e39070a956.

13. Wen ZH, Chao CH, Wu MH, Sheu JH. 2010; A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur J Med Chem. 45:5998–6004. DOI: 10.1016/j.ejmech.2010.09.067. PMID: 20980079.

14. Chen SC, Chien YC, Pan CH, Sheu JH, Chen CY, Wu CH. 2014; Inhibitory effect of dihydroaustrasulfone alcohol on the migration of human non-small cell lung carcinoma A549 cells and the antitumor effect on a Lewis lung carcinoma-bearing tumor model in C57BL/6J mice. Mar Drugs. 12:196–213. DOI: 10.3390/md12010196. PMID: 24413802. PMCID: PMC3917270. PMID: 5bb8f9944e914dbea81e9d80c3dc8670.

15. Hung HC, Feng CW, Lin YY, Chen CH, Tsui KH, Chen WF, Pan CY, Sheu JH, Sung CS, Wen ZH. 2018; Nucleophosmin modulates the alleviation of atopic dermatitis caused by the marine-derived compound dihydroaustrasulfone alcohol. Exp Mol Med. 50:e446. DOI: 10.1038/emm.2017.272. PMID: 29504608. PMCID: PMC5903824.

16. Lin SW, Huang SC, Kuo HM, Chen CH, Ma YL, Chu TH, Bee YS, Wang EM, Wu CY, Sung PJ, Wen ZH, Wu DC, Sheu JH, Tai MH. 2015; Coral-derived compound WA-25 inhibits angiogenesis by attenuating the VEGF/VEGFR2 signaling pathway. Mar Drugs. 13:861–878. DOI: 10.3390/md13020861. PMID: 25668036. PMCID: PMC4344606. PMID: bf30b232d81d4ef1bba9bd8436bf166a.

17. Lu IT, Lin SC, Chu YC, Wen Y, Lin YC, Cheng WC, Sheu JH, Lin CC. 2022; (-)-Agelasidine A induces endoplasmic reticulum stress-dependent apoptosis in human hepatocellular carcinoma. Mar Drugs. 20:109. DOI: 10.3390/md20020109. PMID: 35200638. PMCID: PMC8875608. PMID: a3993e8833984e0aba9daf1ef02e9cbc.

18. Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. 2007; Marine natural products. Nat Prod Rep. 24:31–86. DOI: 10.1039/b603047p. PMID: 17268607.

19. Calcabrini C, Catanzaro E, Bishayee A, Turrini E, Fimognari C. 2017; Marine sponge natural products with anticancer potential: an updated review. Mar Drugs. 15:310. DOI: 10.3390/md15100310. PMID: 29027954. PMCID: PMC5666418. PMID: f02079e829634c7aaed5cd0ff5c1b35b.

20. Rateb ME, Ebel R. 2011; Secondary metabolites of fungi from marine habitats. Nat Prod Rep. 28:290–344. DOI: 10.1039/c0np00061b. PMID: 21229157.

21. Wei J, Gou Z, Wen Y, Luo Q, Huang Z. 2020; Marine compounds targeting the PI3K/Akt signaling pathway in cancer therapy. Biomed Pharmacother. 129:110484. DOI: 10.1016/j.biopha.2020.110484. PMID: 32768966.

22. Ye J, Zhou F, Al-Kareef AM, Wang H. 2015; Anticancer agents from marine sponges. J Asian Nat Prod Res. 17:64–88. DOI: 10.1080/10286020.2014.970535. PMID: 25402340.

23. Guicciardi ME, Gores GJ. 2009; Life and death by death receptors. FASEB J. 23:1625–1637. DOI: 10.1096/fj.08-111005. PMID: 19141537. PMCID: PMC2698650.

24. Schneider-Brachert W, Heigl U, Ehrenschwender M. 2013; Membrane trafficking of death receptors: implications on signalling. Int J Mol Sci. 14:14475–14503. DOI: 10.3390/ijms140714475. PMID: 23852022. PMCID: PMC3742255. PMID: 057d0d23540a44d5b5dcd90bc4ea621c.

25. Wang S, El-Deiry WS. 2003; TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 22:8628–8633. DOI: 10.1038/sj.onc.1207232. PMID: 14634624.

26. Adams JM, Cory S. 1998; The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326. DOI: 10.1126/science.281.5381.1322. PMID: 9735050.

27. Czabotar PE, Lessene G, Strasser A, Adams JM. 2014; Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 15:49–63. DOI: 10.1038/nrm3722. PMID: 24355989.

28. Green DR. 2000; Apoptotic pathways: paper wraps stone blunts scissors. Cell. 102:1–4. DOI: 10.1016/S0092-8674(00)00003-9. PMID: 10929706.
29. Hunt A, Evan G. 2001; Apoptosis. Till death us do part. Science. 293:1784–1785. DOI: 10.1126/science.11546862. PMID: 11546862.
30. Hardwick JM, Soane L. 2013; Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 5:a008722. DOI: 10.1101/cshperspect.a008722. PMID: 23378584. PMCID: PMC3552500.

31. Wu H, Medeiros LJ, Young KH. 2018; Apoptosis signaling and BCL-2 pathways provide opportunities for novel targeted therapeutic strategies in hematologic malignances. Blood Rev. 32:8–28. DOI: 10.1016/j.blre.2017.08.004. PMID: 28802908.

32. Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. 2019; The relation between PI3K/AKT signalling pathway and cancer. Gene. 698:120–128. DOI: 10.1016/j.gene.2019.02.076. PMID: 30849534.

33. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. 2022; Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 80:1–17. DOI: 10.1016/j.semcancer.2019.12.008. PMID: 31866476.

34. Ci Y, Shi K, An J, Yang Y, Hui K, Wu P, Shi L, Xu C. 2014; ROS inhibit autophagy by downregulating ULK1 mediated by the phosphorylation of p53 in selenite-treated NB4 cells. Cell Death Dis. 5:e1542. DOI: 10.1038/cddis.2014.506. PMID: 25429619. PMCID: PMC4260759.

35. Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. 2017; Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med. 104:144–164. DOI: 10.1016/j.freeradbiomed.2017.01.004. PMID: 28088622.

36. Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, Fini M, Russo MA. 2016; The interplay of reactive oxygen species, hypoxia, inflammation, and Sirtuins in cancer initiation and progression. Oxid Med Cell Longev. 2016:3907147. DOI: 10.1155/2016/3907147. PMID: 26798421. PMCID: PMC4699039.

37. Huynh DTN, Jin Y, Myung CS, Heo KS. 2021; Ginsenoside Rh1 induces MCF-7 cell apoptosis and autophagic cell death through ROS-mediated Akt signaling. Cancers (Basel). 13:1892. DOI: 10.3390/cancers13081892. PMID: 33920802. PMCID: PMC8071122. PMID: 3d574bb3dd284183891a415a6d6f849d.

38. Kim SO, Cha HJ, Park C, Lee H, Hong SH, Jeong SJ, Park SH, Kim GY, Leem SH, Jin CY, Hwang EJ, Choi YH. 2019; Cordycepin induces apoptosis in human bladder cancer T24 cells through ROS-dependent inhibition of the PI3K/Akt signaling pathway. Biosci Trends. 13:324–333. DOI: 10.5582/bst.2019.01214. PMID: 31527329.

39. Li X, Zhu F, Jiang J, Sun C, Wang X, Shen M, Tian R, Shi C, Xu M, Peng F, Guo X, Wang M, Qin R. 2015; Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 357:219–230. DOI: 10.1016/j.canlet.2014.11.026. PMID: 25444914.

40. Liu Y, Shi C, He Z, Zhu F, Wang M, He R, Zhao C, Shi X, Zhou M, Pan S, Gao Y, Li X, Qin R. 2021; Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. Int J Biol Sci. 17:589–602. DOI: 10.7150/ijbs.49514. PMID: 33613115. PMCID: PMC7893580.

41. Song X, Wang Z, Liang H, Zhang W, Ye Y, Li H, Hu Y, Zhang Y, Weng H, Lu J, Wang X, Li M, Liu Y, Gu J. 2017; Dioscin induces gallbladder cancer apoptosis by inhibiting ROS-mediated PI3K/AKT signalling. Int J Biol Sci. 13:782–793. DOI: 10.7150/ijbs.18732. PMID: 28656003. PMCID: PMC5485633.

42. Wen C, Wang H, Wu X, He L, Zhou Q, Wang F, Chen S, Huang L, Chen J, Wang H, Ye W, Li W, Yang X, Liu H, Peng J. 2019; ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 10:809. DOI: 10.1038/s41419-019-2035-x. PMID: 31649256. PMCID: PMC6813365.

Fig. 1
The effect of dihydroaustrasulfone alcohol (DA) on cell viability and colony formation in NPC-39 and NPC-BM cells.
Cells were treated with 0.1% DMSO (0 μM) or DA for 24 h. (A) Chemical structure of DA. (B) Cell viability was examined by MTT assays. **p < 0.01, ***p < 0.001 vs. 0.1% DMSO group. Colony formation by (C) NPC-39 and (D) NPC-BM cells following treatment with DA for one week. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. Significant differences compared to the DMSO-treated control group are indicated by *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 2
The effect of dihydroaustrasulfone alcohol (DA) on apoptosis in NPC-39 cells.
Cells were treated with 0.1% DMSO (0 μM) or DA for 24 h (A, C). Cell cycle distribution was determined by propidium iodide (PI) staining and flow cytometry. The means ± SEM of experimental triplicates are shown in the bar graph at the bottom. (B, D) Phosphatidylserine externalization and DNA integrity were determined by FITC-annexin-V and PI, respectively. The lower-right quadrant (annexin-V+/PI−) represents early apoptosis, while the upper-right quadrant (annexin V+/PI+) indicates late apoptosis and necrosis. (E) Expression levels of cleaved poly (ADP-ribose) polymerase (PARP) were investigated by Western blotting using GAPDH as a loading control. (F) Western blot signal intensity quantification. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. Significant differences compared to the DMSO-treated control group are indicated by **p < 0.01, ***p < 0.001.

Fig. 3
The effects of dihydroaustrasulfone alcohol (DA) on caspase-3, caspase-8, and caspase-9 activities in NPC-39 cells.
(A) NPC-39 cells were treated with different concentrations of DA for 24 h, and the activities of (B) caspase-9, (C) caspase-8, and (D) caspase-3 were determined via flow cytometry. Black line: unstained NPC-39 cells, different colored lines: cells treated with different doses of DA. ***p < 0.001 indicates significant differences compared to the DMSO-treated control group. (E) The viability of NPC-39 cells after treatment with a pancaspase inhibitor (Z-VAD-FMK) and DA. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. ***p < 0.001 vs. the 25 μM DA-treated group, as determined by Student’s t-test.
Fig. 4
The effects of dihydroaustrasulfone alcohol (DA) on mitochondrial dysfunction in NPC-39 cells.
Cells were treated with different concentrations of DA for 24 h. (A) Mitochondrial membrane potential (Δψm) and (B) cytochrome c release were determined by JC-1 fluorescent dye staining or anti-cytochrome c–FITC antibodies and flow cytometry. The means ± standard deviation of the experimental triplicates are presented in the bar graph showing (C) JC-1 aggregates and (D) cytochrome c release. All data presented are representative of three independent experiments with similar results. The data are the mean ± standard deviation of three experiments, each performed in triplicate. ***p < 0.001 indicates significant differences compared to the DMSO-treated control group.

Fig. 5
The effects of dihydroaustrasulfone alcohol (DA) on the expression of the Bcl-2 family in NPC-39 cells.
Cells were treated with different concentrations of DA for 24 h. (A) Bcl-2, Bax, and truncated BH3 interacting-domain death agonist (tBid) were examined by Western blot analysis. GAPDH was used as a loading control. (B) Quantitation of the Western blot signal intensities. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. *p < 0.05 and ***p < 0.001 indicate significant differences compared to the DMSO-treated control group.

Fig. 6
The effects of dihydroaustrasulfone alcohol (DA) on the expression of the death receptors DR5, DR4, and FAS in NPC-39 cells.
(A) Flow cytometry was used to evaluate the activity of DR5, DR4, and FAS after the cells were exposed to varying doses of DA for 24 h. (B–D) The bars represent the mean ± standard deviation, and all samples were measured independently in triplicate. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences compared to the DMSO-treated control group. MFI, mean fluorescence intensity.
Fig. 7
The effects of dihydroaustrasulfone alcohol (DA) on the inactivation of the PI3K/AKT signaling pathway in NPC-39 cells.
NPC-39 cells were treated for 24 h with various doses of DA. (A) Cell lysates were collected, and the expression of phosphorylated or nonphosphorylated PI3K and AKT proteins was determined by Western blot analysis. GAPDH was used as a loading control. (B) Western blot signal intensity quantification *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences compared to the DMSO-treated control group. (C) Active AKT cDNA-transfected cells were lysed, and the protein extracts were subjected to protein gel blot analysis using antibodies against the appropriate protein after being treated with 25 µM DA for 24 h. GAPDH was used as a loading control. (D, E) Annexin V-FITC/propidium iodide (PI) binding was investigated using flow cytometry. (F) The MTT assay was used to determine cell viability. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. The difference between the DA and active AKT+ DA groups was **p < 0.01 (Student’s t-test).
Fig. 8
The effects of dihydroaustrasulfone alcohol (DA) on reactive oxygen species (ROS) production in NPC-39 cells.
NPC-39 cells were treated with different concentrations of DA for 1 h, and ROS levels determined by (A, B) flow cytometry. (C) Images were obtained by fluorescence microscopy (original magnification ×200). The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. ***p < 0.001 indicates significant differences compared to the DMSO-treated control group.
Fig. 9
The effects of dihydroaustrasulfone alcohol (DA) on ROS-dependent inactivation of the PI3K/AKT pathway and apoptosis in NPC-39 cells.
After pretreatment with 10 mM NAC for 1 h and then treatment with 25 μM DA for 24 h, cell viability was measured by (A) MTT assays. (B, C) The percentages of apoptotic annexin V+ cells and cell viability were determined by flow cytometry. (D) Cellular proteins were prepared, and the protein expression of pAKT and AKT was evaluated by Western blot analysis and quantitation of the Western blot signal intensities. GAPDH was used as a loading control. The results are presented as the mean ± standard deviation, and all samples were measured independently in triplicate. *p < 0.05, ***p < 0.001 (Student’s t-test) indicates the difference between the DA and NAC + DA groups. ROS, reactive oxygen species; NAC, N-acetylcysteine; PI, propidium iodide.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download