1. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019; 393:1958–1972. PMID:
30954305.
2. Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009; 339:b4567. PMID:
19934192.
3. Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Ah ST, Wei L, Diaz R, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018; 392:496–506. PMID:
30129465.
4. Li Y, Huang Z, Jin C, Xing A, Liu Y, Huangfu C, Lichtenstein AH, Tucker KL, Wu S, Gao X. Longitudinal change of perceived salt intake and stroke risk in a Chinese population. Stroke. 2018; 49:1332–1339. PMID:
29739913.
5. Umesawa M, Iso H, Fujino Y, Kikuchi S, Tamakoshi A, Group JS. JACC Study Group. Salty food preference and intake and risk of gastric cancer: the JACC Study. J Epidemiol. 2016; 26:92–97. PMID:
26477994.
6. Deckers IA, van Engeland M, van den Brandt PA, Van Neste L, Soetekouw PM, Aarts MJ, Baldewijns MM, Keszei AP, Schouten LJ. Promoter CpG island methylation in ion transport mechanisms and associated dietary intakes jointly influence the risk of clear-cell renal cell cancer. Int J Epidemiol. 2017; 46:622–631. PMID:
27789672.
7. Faraco G, Hochrainer K, Segarra SG, Schaeffer S, Santisteban MM, Menon A, Jiang H, Holtzman DM, Anrather J, Iadecola C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. 2019; 574:686–690. PMID:
31645758.
8. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009; 38:791–813. PMID:
19351697.
10. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010; 190:285–296. PMID:
20696704.
11. Bobowski N. Shifting human salty taste preference: potential opportunities and challenges in reducing dietary salt intake of Americans. Chemosens Percept. 2015; 8:112–116. PMID:
26451233.
12. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General [Internet]. Atlanta (GA): Centers for Disease Control and Prevention (US);2014. cited 2021 August 9. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK179276/
.
13. Gao X, Zhang Y, Breitling LP, Brenner H. Tobacco smoking and methylation of genes related to lung cancer development. Oncotarget. 2016; 7:59017–59028. PMID:
27323854.
14. O’Keeffe LM, Taylor G, Huxley RR, Mitchell P, Woodward M, Peters SA. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018; 8:e021611.
15. Lee W, Hwang SH, Choi H, Kim H. The association between smoking or passive smoking and cardiovascular diseases using a Bayesian hierarchical model: based on the 2008–2013 Korea Community Health Survey. Epidemiol Health. 2017; 39:e2017026. PMID:
28728350.
16. Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018; 360:j5855. PMID:
29367388.
17. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015; 3:958–967. PMID:
26388413.
18. Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020; 22:1653–1656. PMID:
32399563.
19. Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, Abedi A, Abhilash ES, Abila DB, Aboyans V, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021; 397:2337–2360. PMID:
34051883.
20. Chen J, Higby R, Tian D, Tan D, Johnson MD, Xiao Y, Kellar KJ, Feng S, Shields PG. Toxicological analysis of low-nicotine and nicotine-free cigarettes. Toxicology. 2008; 249:194–203. PMID:
18599178.
21. Centers for Disease Control and Prevention. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US);2010.
22. Pavlos P, Vasilios N, Antonia A, Dimitrios K, Georgios K, Georgios A. Evaluation of young smokers and non-smokers with electrogustometry and contact endoscopy. BMC Ear Nose Throat Disord. 2009; 9:9. PMID:
19695082.
23. Chéruel F, Jarlier M, Sancho-Garnier H. Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery time-course after smoking cessation. Tob Induc Dis. 2017; 15:15. PMID:
28261024.
24. Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, Sempos CT, Jernigan D. Alcohol as a risk factor for global burden of disease. Eur Addict Res. 2003; 9:157–164. PMID:
12970584.
25. Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OS, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health. 2017; 2:e108–e120. PMID:
29253389.
26. Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, Mittleman MA. Alcohol and immediate risk of cardiovascular events: a systematic review and dose-response meta-analysis. Circulation. 2016; 133:979–987. PMID:
26936862.
27. Larsson SC, Wallin A, Wolk A, Markus HS. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med. 2016; 14:178. PMID:
27881167.
28. Le Berre AP. Emotional processing and social cognition in alcohol use disorder. Neuropsychology. 2019; 33:808–821. PMID:
31448948.
29. Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014; 12:182. PMID:
25567363.
30. Roerecke M, Vafaei A, Hasan OS, Chrystoja BR, Cruz M, Lee R, Neuman MG, Rehm J. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019; 114:1574–1586. PMID:
31464740.
31. Jayasekara H, MacInnis RJ, Room R, English DR. Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: a systematic review and meta-analysis. Alcohol Alcohol. 2016; 51:315–330. PMID:
26400678.
33. Ng GI, Chen CM, Graubard BI, Hoffman HJ, Breslow RA. Alcohol and taste intensity. Chemosens Percept. 2019; 12:90–99.
34. Rose AK, Hardman CA, Christiansen P. The effects of a priming dose of alcohol and drinking environment on snack food intake. Appetite. 2015; 95:341–348. PMID:
26210606.
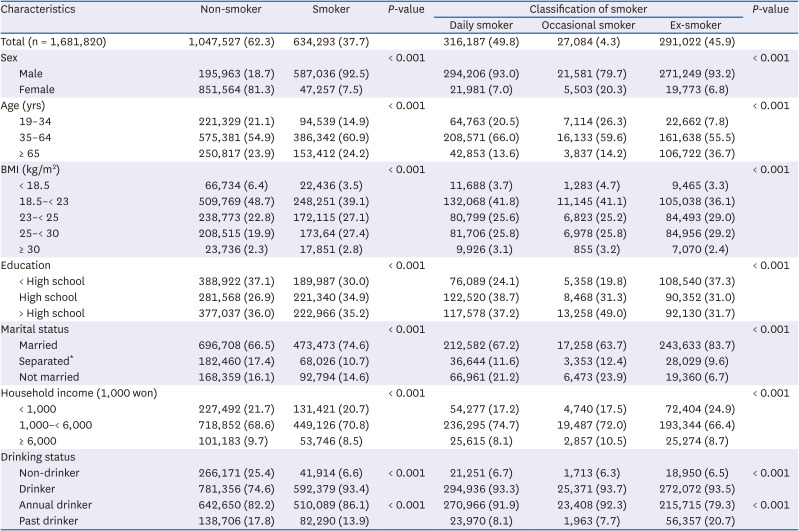
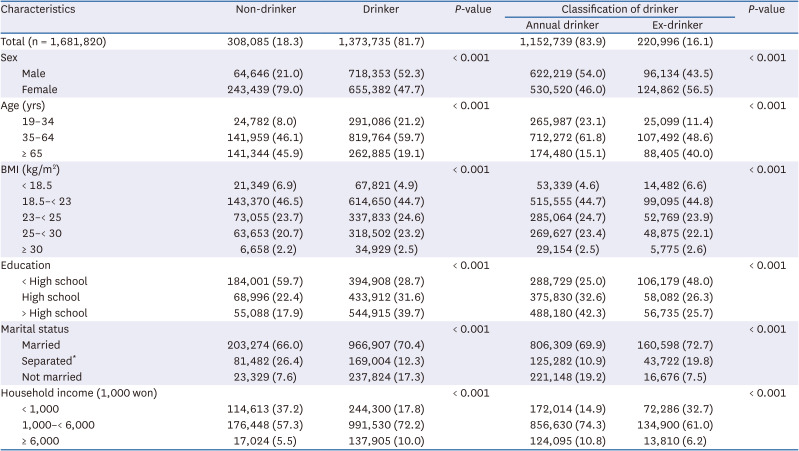
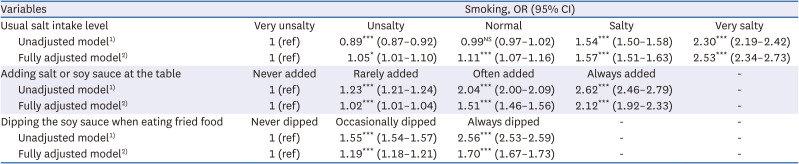
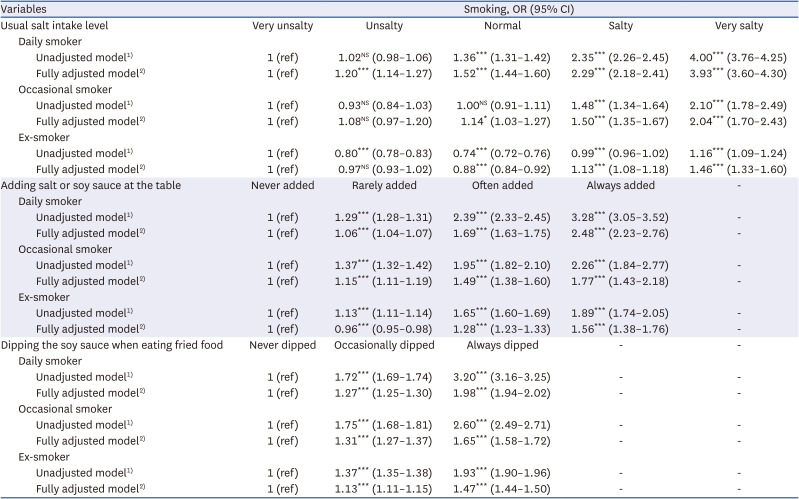
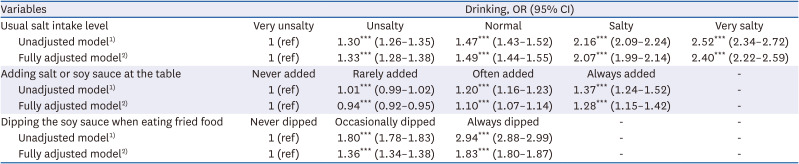
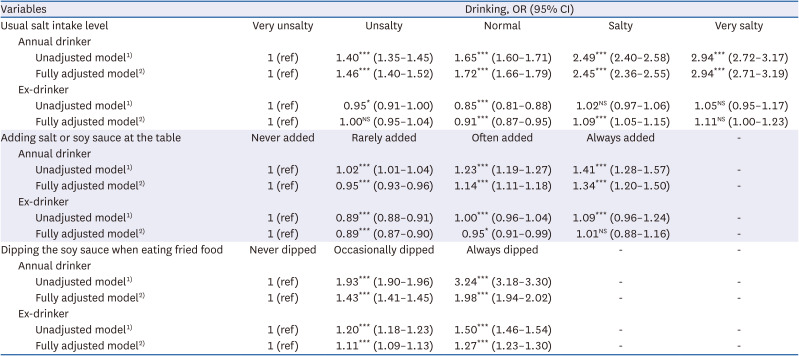
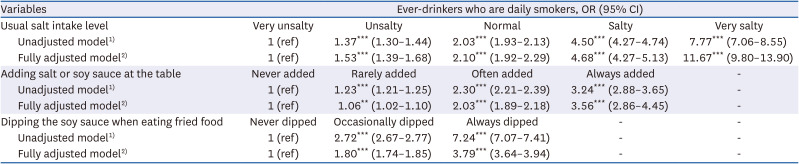
35. Chun IA, Park J, Han MA, Choi SW, Ryu SY. The association between smoking, alcohol intake, and low-salt diet: results from the 2008 Community Health Survey. J Korean Diet Assoc. 2013; 19:223–235.
36. Kim MG, Kim KY, Nam HM, Hong NS, Lee YM. The relationship between lifestyle and sodium intake in Korean middle-aged workers. J Korea Acad Ind Coop Soc. 2014; 15:2923–2929.
37. Lampuré A, Schlich P, Deglaire A, Castetbon K, Péneau S, Hercberg S, Méjean C. Sociodemographic, psychological, and lifestyle characteristics are associated with a liking for salty and sweet tastes in French adults. J Nutr. 2015; 145:587–594. PMID:
25733476.
38. Choi KH, Park MS, Kim JA, Lim JA. Associations between excessive sodium intake and smoking and alcohol intake among Korean men: KNHANES V. Int J Environ Res Public Health. 2015; 12:15540–15549. PMID:
26670236.
39. Romanov RA, Kolesnikov SS. Electrophysiologically identified subpopulations of taste bud cells. Neurosci Lett. 2006; 395:249–254. PMID:
16309836.
40. Bigiani A. Amiloride-sensitive sodium currents in fungiform taste cells of rats chronically exposed to nicotine. Neuroscience. 2015; 284:180–191. PMID:
25305667.
41. Bigiani A. Does ENaC work as sodium taste receptor in humans? Nutrients. 2020; 12:1195. PMID:
32344597.
42. Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006; 96:1877–1886. PMID:
16837661.
43. Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, Desimone JA, Nicolelis MA, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci U S A. 2009; 106:1596–1601. PMID:
19164511.
44. Klein I, Nagler RM, Toffler R, van Der Vliet A, Reznick AZ. Effect of cigarette smoke on oral peroxidase activity in human saliva: role of hydrogen cyanide. Free Radic Biol Med. 2003; 35:1448–1452. PMID:
14642392.
45. Rad M, Kakoie S, Niliye Brojeni F, Pourdamghan N. Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospect. 2010; 4:110–114.
46. Ferragut JM, da Cunha MR, Carvalho CA, Isayama RN, Caldeira EJ. Epithelial-stromal interactions in salivary glands of rats exposed to chronic passive smoking. Arch Oral Biol. 2011; 56:580–587. PMID:
21168828.
47. Dyasanoor S, Saddu SC. Association of xerostomia and assessment of salivary flow using modified schirmer test among smokers and healthy individuals: a preliminutesary study. J Clin Diagn Res. 2014; 8:211–213.
48. Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980; 13:349–357. PMID:
7428010.
49. Bartoshuk LM, Sims CA, Colquhoun TA, Snyder DJ. What Aristotle didn’t know about flavor. Am Psychol. 2019; 74:1003–1011. PMID:
31829675.
50. Snyder DJ, Bartoshuk LM. Oral sensory nerve damage: causes and consequences. Rev Endocr Metab Disord. 2016; 17:149–158. PMID:
27511471.
51. Schneller LM, McIntosh S, Li D, Rahman I, Ossip D, Goniewicz M, O’Connor RJ. Tobacco use and chemosensory impairments among current adult tobacco users in the US: data from NHANES 2013-2014. Tob Induc Dis. 2018; 16:43. PMID:
31516441.
52. Yeon JY, Kim EY, Lee EJ, Bae YJ. Relationship among pack-years of smoking, metabolic biomarkers, and diet quality in male adults: From the Korean national health and nutrition examination surveys, 2007–2009. J East Asian Soc Diet Life. 2012; 22:175–189.
53. Alkerwi A, Baydarlioglu B, Sauvageot N, Stranges S, Lemmens P, Shivappa N, Hébert JR. Smoking status is inversely associated with overall diet quality: findings from the ORISCAV-LUX study. Clin Nutr. 2017; 36:1275–1282. PMID:
27595637.
54. Kloss L, Meyer JD, Graeve L, Vetter W. Sodium intake and its reduction by food reformulation in the European Union — a review. NFS J. 2015; 1:9–19.
55. Méjean C, Macouillard P, Castetbon K, Kesse-Guyot E, Hercberg S. Socio-economic, demographic, lifestyle and health characteristics associated with consumption of fatty-sweetened and fatty-salted foods in middle-aged French adults. Br J Nutr. 2011; 105:776–786. PMID:
20946706.
56. Terry JG, Hartley KG, Steffen LM, Nair S, Alman AC, Wellons MF, Jacobs DR Jr, Tindle HA, Carr JJ. Association of smoking with abdominal adipose deposition and muscle composition in coronary artery risk development in young adults (CARDIA) participants at mid-life: a population-based cohort study. PLoS Med. 2020; 17:e1003223. PMID:
32692748.
57. Travier N, Agudo A, May AM, Gonzalez C, Luan J, Wareham NJ, Bueno-de-Mesquita HB, van den Berg SW, Slimani N, Rinaldi S, et al. Longitudinal changes in weight in relation to smoking cessation in participants of the EPIC-PANACEA study. Prev Med. 2012; 54:183–192. PMID:
21939684.
58. Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012; 345:e4439. PMID:
22782848.
59. Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One. 2015; 10:e0123579. PMID:
25886648.
60. Kim JH, Shim KW, Yoon YS, Lee SY, Kim SS, Oh SW. Cigarette smoking increases abdominal and visceral obesity but not overall fatness: an observational study. PLoS One. 2012; 7:e45815. PMID:
23029258.
61. Nakanishi K, Nishida M, Ohama T, Moriyama T, Yamauchi-Takihara K. Smoking associates with visceral fat accumulation especially in women. Circ J. 2014; 78:1259–1263. PMID:
24621566.
62. Komiya H, Mori Y, Yokose T, Tajima N. Smoking as a risk factor for visceral fat accumulation in Japanese men. Tohoku J Exp Med. 2006; 208:123–132. PMID:
16434835.
63. Carreras-Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, Martin RM. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ. 2018; 361:k1767. PMID:
29769355.
64. Zhang X, Wang J, Li J, Yu Y, Song Y. A positive association between dietary sodium intake and obesity and central obesity: results from the National Health and Nutrition Examination Survey 1999–2006. Nutr Res. 2018; 55:33–44. PMID:
29914626.
65. Larsen BA, Litt MD, Huedo-Medina TB, Duffy VB. Modeling associations between chemosensation, liking for fats and sweets, dietary behaviors and body mass index in chronic smokers. Nutrients. 2019; 11:E271.
66. Anker JJ, Nakajima M, Raatz S, Allen S, al’Absi M. Tobacco withdrawal increases junk food intake: the role of the endogenous opioid system. Drug Alcohol Depend. 2021; 225:108819. PMID:
34182373.
68. Thibodeau M, Bajec M, Pickering G. Orosensory responsiveness and alcohol behaviour. Physiol Behav. 2017; 177:91–98. PMID:
28433466.
69. Silva CS, Dias VR, Almeida JA, Brazil JM, Santos RA, Milagres MP. Effect of heavy consumption of alcoholic beverages on the perception of sweet and salty taste. Alcohol Alcohol. 2016; 51:302–306. PMID:
26464460.
70. Schrieks IC, Stafleu A, Griffioen-Roose S, de Graaf C, Witkamp RF, Boerrigter-Rijneveld R, Hendriks HF. Moderate alcohol consumption stimulates food intake and food reward of savoury foods. Appetite. 2015; 89:77–83. PMID:
25636235.
71. Hayes JE, Sullivan BS, Duffy VB. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol Behav. 2010; 100:369–380. PMID:
20380843.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download