INTRODUCTION
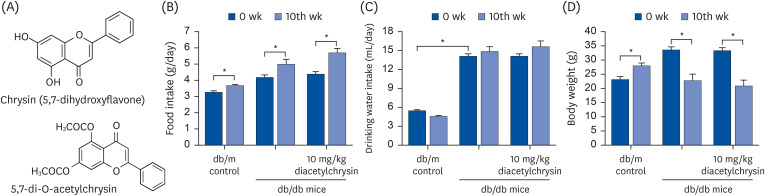 | Fig. 1Chemical structures of chrysin and diacetylchrysin (A), and changes in food intake (B), drinking water intake (C), and body weight (D) of db/m controls and db/db mice after the 10 week-supplementing challenge with diacetylchrysin. The db/db mice were supplemented with 10 mg/kg diacetylchrysin for 10 weeks. The db/m mice were introduced as control animals. The data are presented as mean ± SEM for each treatment group (n = 9).SEM, standard error of mean.
*P < 0.05 relative to 10th week or db/m control mice.
|
MATERIALS AND METHODS
Materials
Synthesis of 5,7-di-O-acethylchrysin
In vivo animal experiments
Sampling of blood and urine
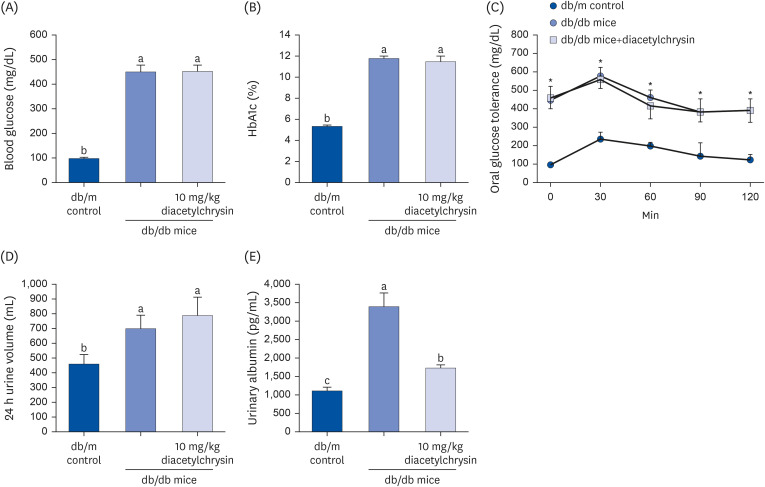 | Fig. 2Blood levels of fasting glucose (A) and glycated hemoglobin level (HbA1c, B), oral glucose tolerance curve (C), 24 h urine volume (D), and urinary albumin excretion (E) of db/db mice treated with diacetylchrysin. The db/db mice were supplemented with 10 mg/kg diacetylchrysin for 10 weeks. The db/m mice were introduced as control animals. The blood glucose levels and HbA1c, 24 h urine volume, and urinary albumin excretion were measured after the 10 weeks treatment of diacetylchrysin. The values (mean ± SEM, n = 9) not sharing a common small letter are significantly different at P < 0.05.HbA1C, hemoglobin A1c; SEM, standard error of mean.
*P < 0.05 relative to db/m control mice.
|
Masson’s trichrome staining
Western blot analysis
Periodic acid-Schiff (PAS) staining
Immunohistochemical staining
FITC-dextran perfused retinal flat mounts
Retinal trypsin digestion assay
Statistical analysis
RESULTS
Inhibition of collagen accumulation by diacetylchrysin
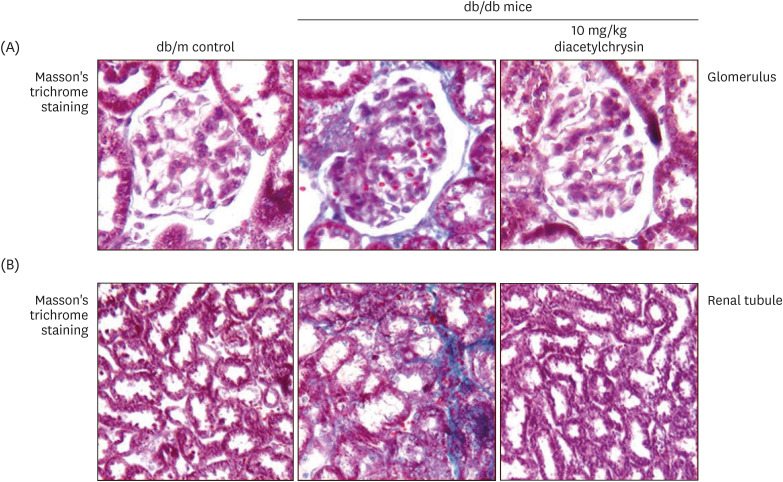 | Fig. 3Effects of diacetylchrysin on formation of collagen fibers in glomeruli (A) and renal tubules (B). The db/db mice were supplemented with 10 mg/kg diacetylchrysin for 10 weeks. The db/m mice were employed as control animals. Glomerular and tubular fibrosis in db/db mouse kidneys was observed by Masson’s trichrome staining (A and B). The collagen fibers were stained in blue. Each photograph is representative of 4 mice. Magnification: 200-fold. |
Effects of diacetylchrysin on induction of ECM-related enzymes
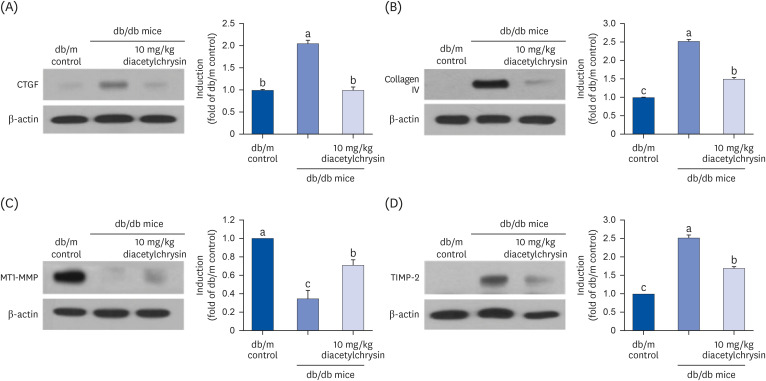 | Fig. 4Effects of diacetylchrysin on the expressions of CTGF (A), collagen IV (B), MT1-MMP (C), and TIMP-2 (D) in db/db mice. The db/db mice were orally treated with 10 mg/kg diacetylchrysin or 10 mg/kg chrysin for 10 weeks. The db/m mice were introduced as control animals. Western blot analysis with tissue extracts was conducted with a primary antibody against CTGF, collagen IV, MT1-MMP, and TIMP-2. β-Actin protein was used as an internal control. The bar graphs (mean ± SEM, n = 3) in the right panel represent quantitative results obtained from a densitometer. Values not sharing a common small letter are significantly different at P < 0.05.CTGF, connective tissue growth factor; MT1-MMP, membrane type 1-matrix metalloproteinase; TIMP-2, tissue inhibitor of metalloproteinase 2; SEM, standard error of mean.
|
Inhibitory effects of diacetylchrysin on glomerular sclerosis and tubular injury
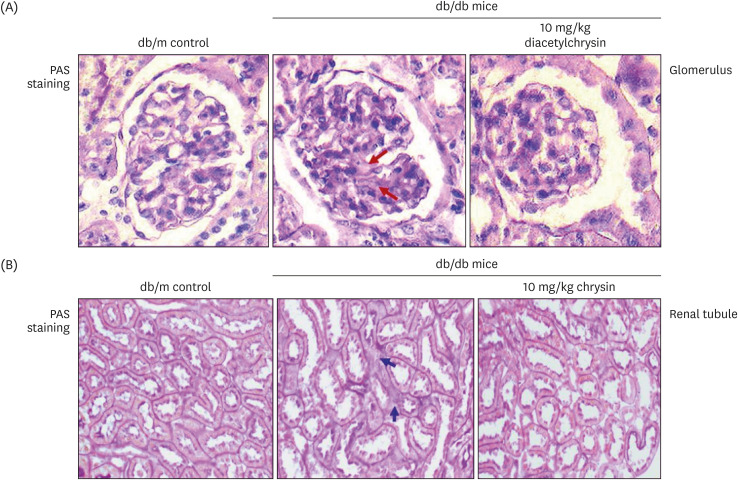 | Fig. 5Inhibition of renal interstitial fibrosis (arrows) in glomeruli (A) and renal tubules (B) of db/db mice by diacetylchrysin. The db/db mice were supplemented with 10 mg/kg diacetylchrysin for 10 weeks. The db/m mice were employed as control animals. Glomerular and tubular tissue sections were stained by PAS reagent and counterstained with hematoxylin (A and B). Each photograph is representative of four mice. Magnification: 200-fold.PAS, Periodic acid-Schiff.
|
Induction of retinal endothelial proteins by diacetylchrysin
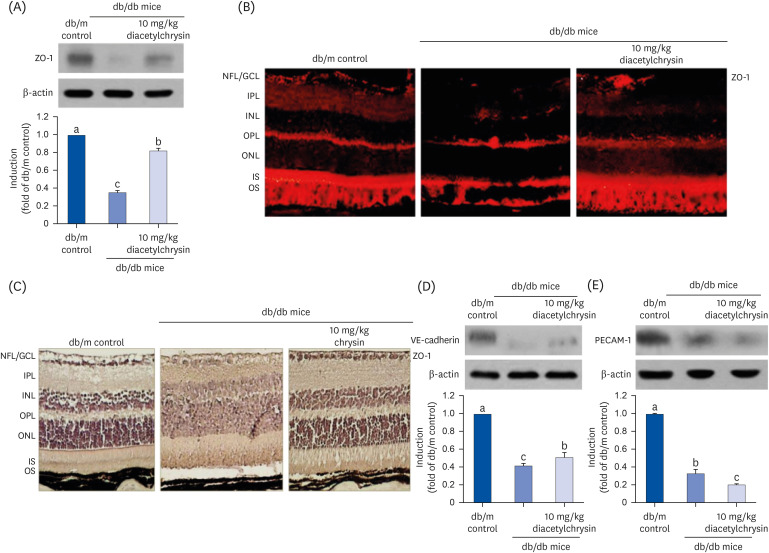 | Fig. 6Effects of diacetylchrysin on the expression of endothelial junction markers. The db/db mice were orally supplemented with 10 mg/kg diacetylchrysin daily for 10 weeks. The db/m mice were introduced as control animals. Mouse retinal tissue extracts were subject to Western blot analysis with a primary antibody against ZO-1 (A), VE-cadherin (D), and PECAM-1 (E). β-Actin protein was used as an internal control. Bar graphs (mean ± SEM, n = 3) in the right panel represent densitometric results of left blot bands. Values not sharing a common small letter differ, P < 0.05. Histological sections of mouse retina were immunohistochemically stained using a primary antibody of ZO-1 and red Cy3-conjugated secondary antibody for visualizing the ZO-1 induction (B). In another experiment with 10 mg/kg chrysin administration, the ZO-1 expression was identified as 3,3′-diaminobenzidine staining (brown) and the sections were counterstained with hematoxylin (C). Each photograph is representative of four mice. Magnification: 200-fold. Retinal layers are labeled as follows: NFL/GCL, IPL, INL, OPL, ONL, and photoreceptor IS/OS.ZO, Zona occluden; VE, vascular endothelial; PECAM, platelet endothelial adhesion molecule; SEM, standard error of mean; NFL, neurofiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment.
|
Inhibition of retinal neovascularization by diacetylchrysin
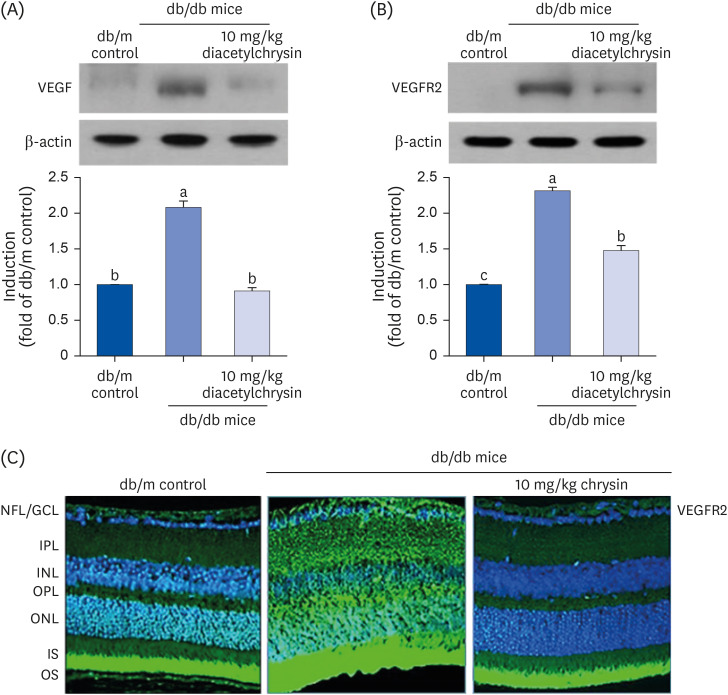 | Fig. 7Inhibition of retinal tissue induction of VEGF (A) and VEGFR2 (B) by diacetylchrysin. The db/db mice were orally administered 10 mg/kg diacetylchrysin daily for 10 weeks. The db/m mice were employed as control animals. Mouse retinal tissue extracts were subject to Western blot analysis with a primary antibody against VEGF and VEGFR2 (A and B, respectively). β-Actin protein was used as an internal control. Bar graphs (mean ± SEM, n = 3) in the right panel represent densitometric results of left blot bands. Values not sharing a common small letter differ, P < 0.05. Immunocytochemical analysis of VEGFR2 in db/db mice treated with 10 mg/kg chrysin was achieved by histological staining of mouse retina sections using a primary antibody of VEGFR2. FITC-conjugated secondary antibody was used for visualizing the VEGFR2, and the nuclear staining was done with 4′,6-diamidino-2-phenylindole (C). Each photograph is representative of 4 mice. Magnification: 200-fold. Retinal layers are labeled as follows: NFL/GCL, IPL, INL, OPL, ONL, and photoreceptor IS/OS.VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; SEM, standard error of mean; FITC, fluorescein isothiocyanate; NFL, neurofiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment.
|
Suppressive effect of diacetylchrysin on retinal vessel leakage
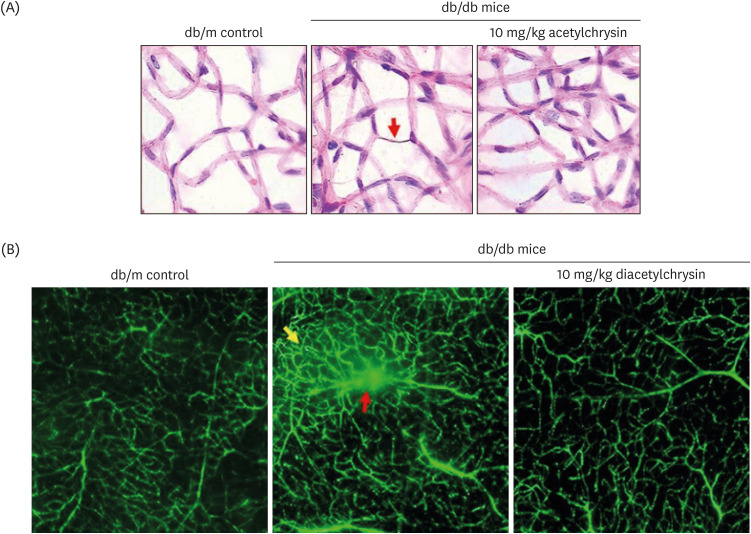 | Fig. 8Inhibition of abnormal neovascularization by diacetylchrysin. The db/db mice were orally supplemented with 10 mg/kg diacetylchrysin daily for 10 weeks. The db/m mice were employed as control animals. To reveal acellular capillaries (red arrow) in db/db mice treated with 10 mg/kg diacetylchrysin, retinal vessels were subjected to H&E staining (A). Magnification: 200-fold. To detect new capillary bed formation (yellow arrow) and vessel leakage (red arrow) by diacetylchrysin exposure (B), typical appearance of retinal capillaries was observed by FITC-dextran-perfused retinal flat-mounts. Retinas were dissected, flat mounted, and observed by confocal microscopy. Magnification: 400-fold. Each photograph is representative of 4 mice.H&E, hematoxylin and eosin; FITC, fluorescein isothiocyanate.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download