Introduction
Methods
Standardized reporting and registration
Data sources and searches
Study selection
Population
Intervention
Outcomes
Study designs
Screening
Data collection
Risk of bias assessment
Data synthesis and analysis
Certainty of evidence assessment
Living review and literature surveillance
Results
Figure 1.
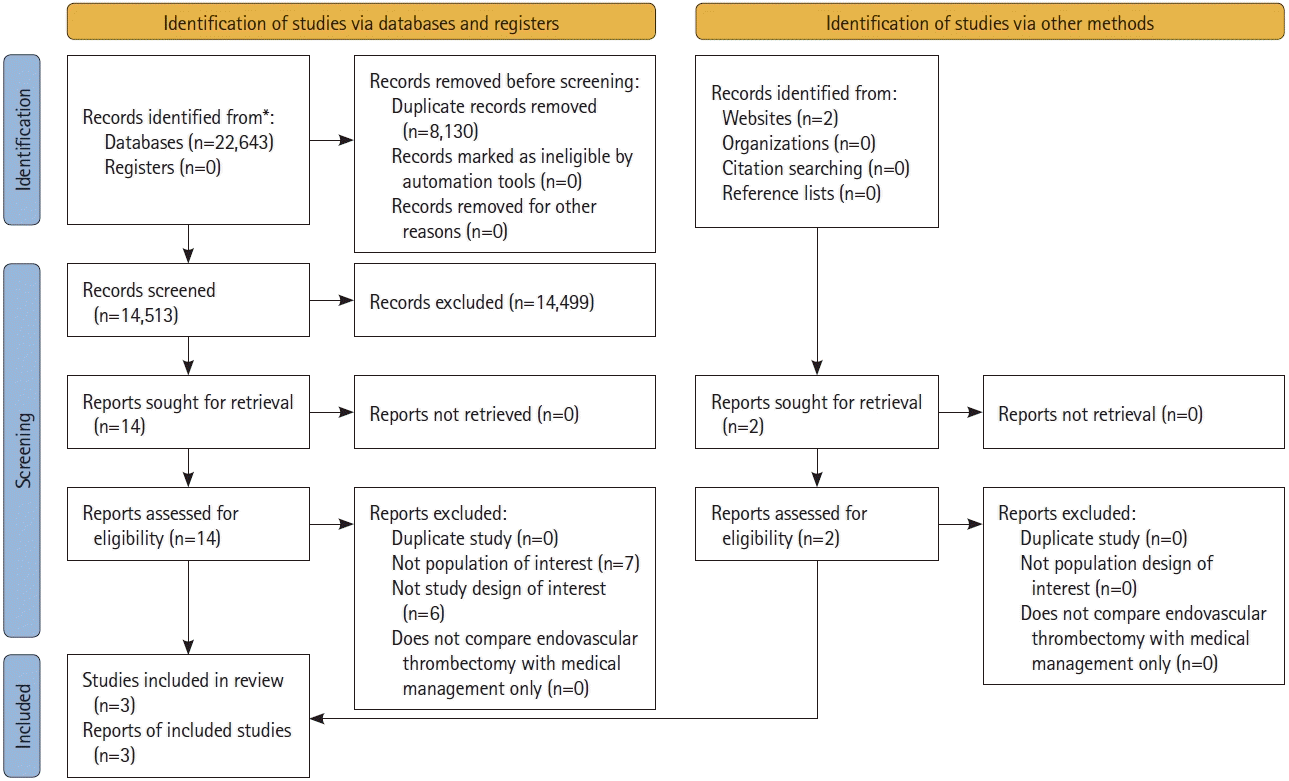
Characteristics of included studies
Table 1.
| Study ID | Country | Study timeframe | Study design | Sample size (n) | Age, y (mean, SD) | Male (%) | History of stroke (%) | History of atrial fibrillation (%) | History of diabetes (%) | History of hypertension (%) | Initial NIHSS (mean, SD) | Initial ASPECTS (mean, SD) | Infarct core volume, mL (mean, SD) | ICA occlusion (%) | M1 MCA occlusion (%) | M2 MCA occlusion (%) | Tandem occlusion (%) | Intravenous thrombolysis (%) | Type and dose of thrombolytic agent used |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huo 2023 [7] [ANGEL- ASPECT] | China | October 2, 2020–May 18, 2022 | RCT | 455 | 66.84 (9.72) | 61.32 | 16.04 | 22.86 | 18.24 | 59.78 | 16 (5) | 3 (1) | 59.24 (41.76) | 36.04 | 63.08 | 0.88 | 16.70 | 28.35 | Alteplase 0.9 mg/kg; urokinase 1.0 to 1.5 million IU |
| Sarraj 2023 [8] [SELECT-2] | International | September 2019–September 2022 | RCT | 352 | 66.50 (12.69) | 58.81 | 9.09 | 24.15 | 30.68 | 73.86 | 19 (6) | 4 (1) | 84.76 (41.35) | 41.48 | 54.26 | 4.26 | 28.41 | 19.09 | Alteplase NOS; tenecteplase NOS |
| Yoshimura 2022 [9] [RESCUE-Japan LIMIT] | Japan | November 2018–September 2021 | RCT | 203 | 76.15 (10.09) | 55.67 | 25.12 | 59.11 | 21.18 | 69.46 | 22 (6) | 4 (1) | 106.01 (57.50) | 47.29 | 70.94 | 1.48 | 19.70 | 27.59 | Alteplase 0.6 mg/kg |
SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; MCA, middle cerebral artery; RCT, randomized controlled trial; IU, international unit; NOS, not otherwise specified; ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT-2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial.
Risk of bias assessment
Outcomes for EVT versus medical management only
Functional independence (mRS score 0–2 at 90 days)
Figure 2.
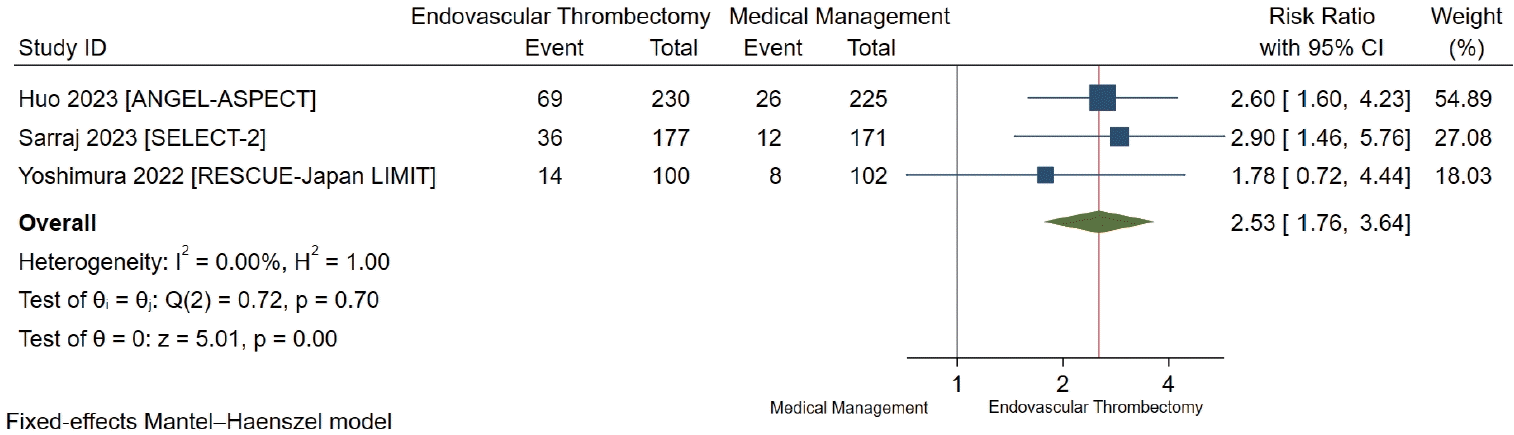
Table 2.
| Outcomes of all RCTs [7-9] |
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inconsistency | Indirectness | Imprecision | Endovascular therapy | Medical management only | RR (95% CI) | Baseline risk for control group (%)* | RD (%) (95% CI) | |||
| Functional independence at 90 days | Not serious | Not serious | Very serious† | 119/507 (23.5%) | 46/498 (9.2%) | 2.53 (1.76 to 3.64) | 19.8 | 30.3 (15.0 to 52.3) | ⊕⊕◯◯ | CRITICAL |
| Low | ||||||||||
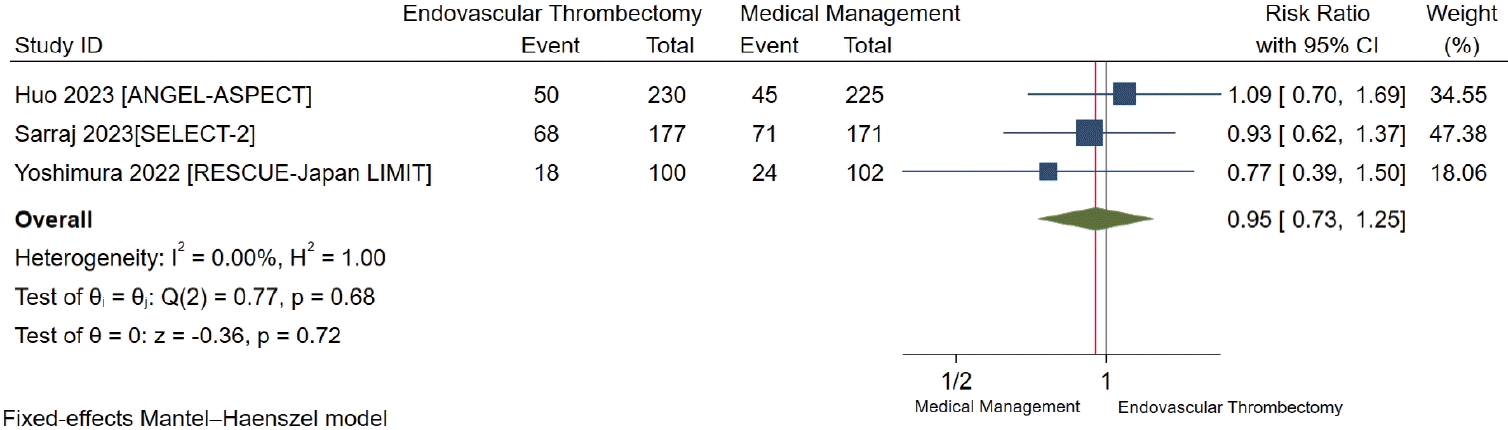
| Mortality at 90 days | Not serious | Not serious | Very serious† | 136/507 (26.8%) | 140/498 (28.1%) | 0.95 (0.73 to 1.25) | 14.0 | -0.7 (-3.8 to 3.5) | ⊕⊕◯◯ | CRITICAL |
| Low | ||||||||||
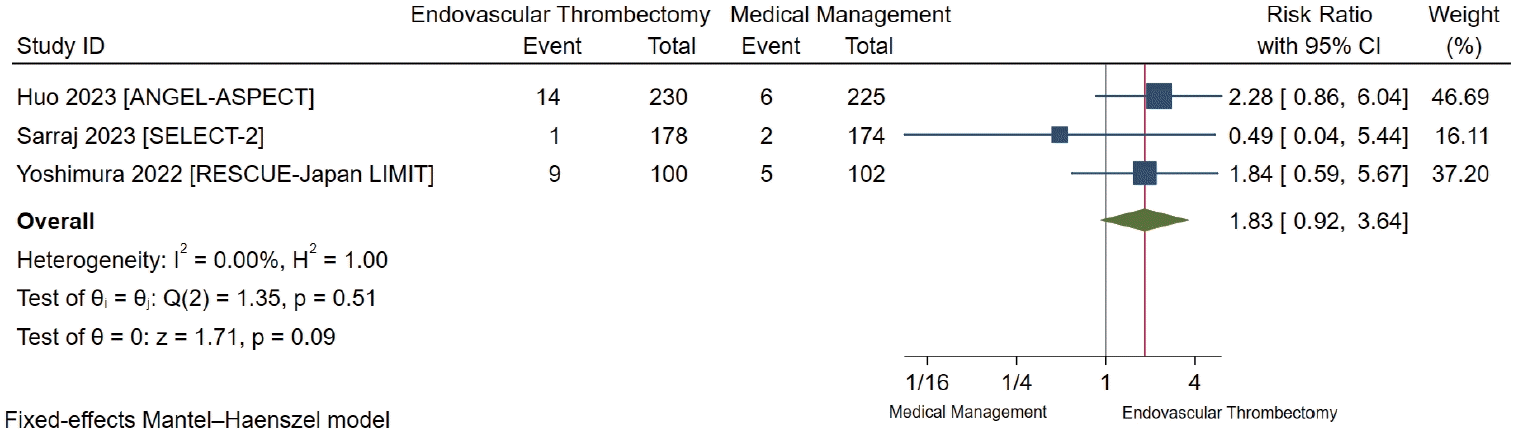
| Symptomatic intracranial hemorrhage | Not serious | Not serious | Very serious† | 24/508 (4.7%) | 13/501 (2.6%) | 1.83 (0.92 to 3.64) | 3.7 | 3.1 (-0.3 to 9.8) | ⊕⊕◯◯ | IMPORTANT |
| Low | ||||||||||
GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; RCTs, randomized controlled trials; EVT, endovascular thrombectomy; RR, risk ratio; CI, confidence interval; RD, risk difference.
Mortality at 90 days
Figure 3.
Symptomatic intracranial hemorrhage
Figure 4.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download