Abstract
Background and Purpose
Methods
Results
Conclusion
Supplementary materials
Supplementary Table 1.
Notes
Conflicts of interest
The disclosures of conflict of interest of all authors are provided in Appendix 2.
Author contribution
Conceptualization: CT, STE, HG. Study design: CT, STE, HG. Methodology: CT, STE, HG. Data collection: all authors. Investigation: all authors. Statistical analysis: CT, JL, HG. Writing—original draft: CT, STE, HG. Writing—review & editing: all authors. Approval of final manuscript: all authors.
References
Figure 1.
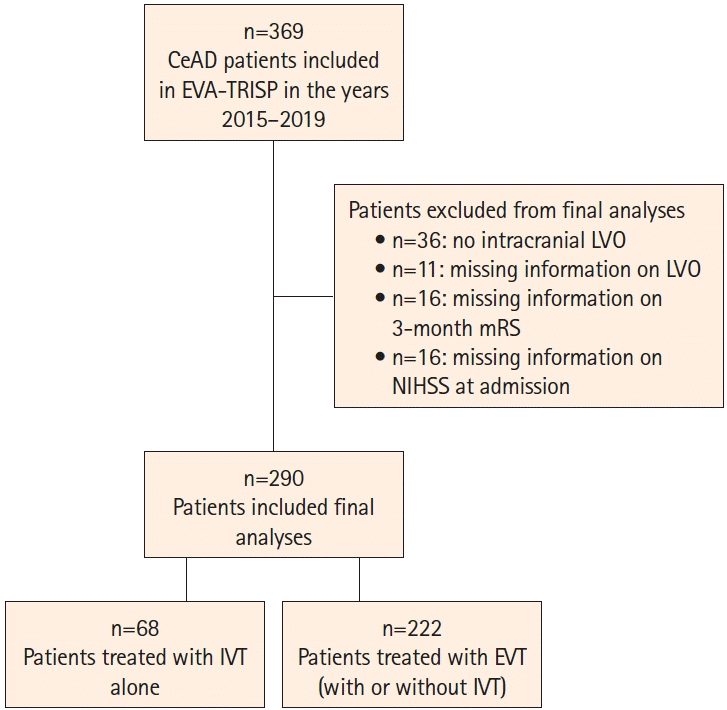
Table 1.
Table 2.
Table 3.
| EVT (n=222) | IVT (n=68) |
Unadjusted (EVT vs. IVT) |
Adjusted (EVT vs. IVT) |
||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Primary outcomes | |||||||
| Favorable outcome (mRS 0–2) | 142/222 (64) | 59/68 (86.8) | 0.27 (0.13–0.58) | 0.001 | 0.56 (0.24–1.32) | 0.183* | |
| Complete recanalization (TICI 2b/3) | 161/200 (80.5) | 22/54 (40.7) | 6.01 (3.15–11.45) | <0.001 | 8.85 (4.28–18.29) | <0.001† | |
| Secondary outcomes | |||||||
| Early neurological improvement | 95/222 (42.8) | 41/68 (60.3) | 0.49 (0.28–0.86) | 0.012 | 0.59 (0.31–1.14) | 0.118* | |
| Symptomatic ICH (ECASS) | 11/220 (5.0) | 1/68 (1.5) | 3.53 (0.45–27.82) | 0.306 | NA | NA | |
| Any ICH | 59/215 (27.4) | 6/66 (9.1) | 3.78 (1.55–9.22) | 0.002 | NA | NA | |
| Death | 16/222 (7.2) | 3/68 (4.4) | 1.68 (0.48–5.96) | 0.578 | NA | NA | |
Data are presented as n (%) unless otherwise indicated.
EVT, endovascular treatment; IVT, intravenous thrombolysis; OR, odds ratio; CI, confidence interval; mRS, modified Rankin Scale; TICI, thrombolysis in cerebral infarction; NA, no adjusted analyses due to low number of events in IVT-group; ICH, intracranial hemorrhage; ECASS, European Cooperative Acute Stroke Study.
Table 4.
Appendices
Appendix 1.
STROBE Statement—Checklist of items that should be included in reports of cohort studies
| Item no | Recommendation | Page no | ||
|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1, 2 | |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | ||||
| Introduction | ||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 2 | |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2 | |
| Methods | ||||
| Study design | 4 | Present key elements of study design early in the paper | 2, 3 | |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 2, 3 | |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 3 | |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | ||||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 3 | |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 3 | |
| Bias | 9 | Describe any efforts to address potential sources of bias | 7, 8 | |
| Study size | 10 | Explain how the study size was arrived at | 4 | |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | NA | |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 3, 4 | |
| (b) Describe any methods used to examine subgroups and interactions | ||||
| (c) Explain how missing data were addressed | ||||
| (d) If applicable, explain how loss to follow-up was addressed | ||||
| (e) Describe any sensitivity analyses | ||||
| Results | ||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 4, ff | |
| (b) Give reasons for non-participation at each stage | ||||
| (c) Consider use of a flow diagram | ||||
| Descriptive data | 14* | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 4, ff | |
| (b) Indicate number of participants with missing data for each variable of interest | ||||
| (c) Summarise follow-up time (e.g., average and total amount) | ||||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | 6 | |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 4, ff | |
| (b) Report category boundaries when continuous variables were categorized | ||||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 6 | |
| Discussion | ||||
| Key results | 18 | Summarise key results with reference to study objectives | 7 | |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 8 | |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 8 | |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 8 | |
| Other information | ||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 8 | |
An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting.
The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download