1. Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020; 126(10):2225–2249. PMID:
32162336.
2. Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990-2019. Gynecol Oncol. 2021; 161(2):573–580. PMID:
33551200.
3. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016; 387(10023):1094–1108. PMID:
26354523.
4. Koskas M, Amant F, Mirza MR, Creutzberg CL. Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet. 2021; 155:Suppl 1. (Suppl 1):45–60. PMID:
34669196.
5. Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol. 2010; 116(3):399–403. PMID:
20022094.
6. Kolehmainen AM, Pasanen A, Tuomi T, Koivisto-Korander R, Butzow R, Loukovaara M. American Society of Anesthesiologists physical status score as a predictor of long-term outcome in women with endometrial cancer. Int J Gynecol Cancer. 2019; 29(5):ijgc-2018-000118.
7. Driver JA, Viswanathan AN. Frailty measure is more predictive of outcomes after curative therapy for endometrial cancer than traditional risk factors in women 60 and older. Gynecol Oncol. 2017; 145(3):526–530. PMID:
28359689.
8. Pinar Cilesiz Goksedef B, Gorgen H, Baran SY, Api M, Cetin A. Preoperative serum CA 125 level as a predictor for metastasis and survival in endometrioid endometrial cancer. J Obstet Gynaecol Can. 2011; 33(8):844–850. PMID:
21846440.
9. Ekici H, Malatyalioglu E, Kokcu A, Kurtoglu E, Tosun M, Celik H. Do leukocyte and platelet counts have benefit for evaluation of endometrial cancer? Asian Pac J Cancer Prev. 2015; 16(13):5305–5310. PMID:
26225670.
10. Reijnen C, Visser NC, Kasius JC, Boll D, Geomini PM, Ngo H, et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: a multicenter prospective cohort study. J Gynecol Oncol. 2019; 30(5):e70. PMID:
31328454.
11. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013; 37(11):2688–2692. PMID:
23884382.
12. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nippon Geka Gakkai Zasshi. 1984; 85(9):1001–1005. PMID:
6438478.
13. Komura N, Mabuchi S, Yokoi E, Shimura K, Kawano M, Matsumoto Y, et al. Prognostic significance of the pretreatment prognostic nutritional index in patients with epithelial ovarian cancer. Oncotarget. 2019; 10(38):3605–3613. PMID:
31217896.
14. Zhang W, Ye B, Liang W, Ren Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep. 2017; 7(1):9548. PMID:
28842710.
15. Haraga J, Nakamura K, Omichi C, Nishida T, Haruma T, Kusumoto T, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol. 2016; 5(5):567–574. PMID:
27900086.
16. Kiuchi K, Hasegawa K, Ochiai S, Motegi E, Kuno T, Kosaka N, et al. Prognostic significance of inflammatory parameters and nutritional index in clinical stage IVB endometrial carcinomas. J Obstet Gynaecol. 2019; 39(2):237–241. PMID:
30370797.
17. Mirili C, Bilici M. Inflammatory prognostic markers in endometrial carcinoma: Systemic immune-inflammation index and prognostic nutritional index. Med Sci Discov. 2020; 7(1):351–359.
18. Njoku K, Barr CE, Ramchander NC, Crosbie EJ. Impact of pre-treatment prognostic nutritional index and the haemoglobin, albumin, lymphocyte and platelet (HALP) score on endometrial cancer survival: a prospective database analysis. PLoS One. 2022; 17(8):e0272232. PMID:
35925991.
19. Kim NR, So KA, Kim TJ, Lim K, Lee KH, Kim MK. Role of systematic lymphadenectomy in patients with intermediate to high-risk early stage endometrial cancer. J Gynecol Oncol. 2023; 34:e23. PMID:
36562131.
20. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106(6):dju124. PMID:
24875653.
21. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014; 20(23):6212–6222. PMID:
25271081.
22. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019; 367:l5657. PMID:
31645336.
23. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71(3):209–249. PMID:
33538338.
24. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999-2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64(5):444–453. PMID:
34399564.
25. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70(1):7–30. PMID:
31912902.
26. Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009; 15(2):425–430. PMID:
19147746.
27. Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013; 114(1):149–154. PMID:
23780645.
28. Sheehan KM, Sheahan K, O’Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999; 282(13):1254–1257. PMID:
10517428.
29. Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999; 13(11):1382–1397. PMID:
10364156.
30. Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002; 51(6):293–298. PMID:
12111117.
31. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009; 30(7):1073–1081. PMID:
19468060.
32. Patel MV, Shen Z, Rodriguez-Garcia M, Usherwood EJ, Tafe LJ, Wira CR. Endometrial cancer suppresses CD8+ T cell-mediated cytotoxicity in postmenopausal women. Front Immunol. 2021; 12:657326. PMID:
33968059.
33. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020; 133(6):713–722.e7. PMID:
31751531.
34. Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. 2006; 30(5):453–463. PMID:
16931617.
35. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013; 58(5):1836–1846. PMID:
23423799.
36. MacDonald N. . Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol. 2007; 5(4):157–162. PMID:
17500503.
37. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998; 32(6):Suppl 4. S118–S125.
38. Gelin J, Moldawer LL, Lönnroth C, Sherry B, Chizzonite R, Lundholm K. Role of endogenous tumor necrosis factor α and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991; 51(1):415–421. PMID:
1703040.
39. Aoyama T, Takano M, Miyamoto M, Yoshikawa T, Kato K, Sakamoto T, et al. Pretreatment neutrophil-to-lymphocyte ratio was a predictor of lymph node metastasis in endometrial cancer patients. Oncology. 2019; 96(5):259–267. PMID:
30893700.
40. Ni L, Tao J, Xu J, Yuan X, Long Y, Yu N, et al. Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020; 301(1):251–261. PMID:
31768743.
41. Dong Y, Cheng Y, Wang J. The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer. Open Life Sci. 2019; 14(1):110–118. PMID:
33817142.
42. Haruma T, Nakamura K, Nishida T, Ogawa C, Kusumoto T, Seki N, et al. Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer Res. 2015; 35(1):337–343. PMID:
25550569.
43. Matsubara S, Mabuchi S, Takeda Y, Kawahara N, Kobayashi H. Prognostic value of pre-treatment systemic immune-inflammation index in patients with endometrial cancer. PLoS One. 2021; 16(5):e0248871. PMID:
33989285.
44. Son J, Carr C, Yao M, Radeva M, Priyadarshini A, Marquard J, et al. Endometrial cancer in young women: prognostic factors and treatment outcomes in women aged ≤40 years. Int J Gynecol Cancer. 2020; 30(5):631–639. PMID:
32213530.
45. Persson I, Adami HO, Malker B, Pettersson B. Long-term survival in endometrial cancer with special reference to age as a prognostic factor. Ups J Med Sci. 1984; 89(2):159–170. PMID:
6464244.
46. Chang SJ, Kim WY, Yoon JH, Yoo SC, Chang KH, Ryu HS. Para-aortic lymphadenectomy improves survival in patients with intermediate to high-risk endometrial carcinoma. Acta Obstet Gynecol Scand. 2008; 87(12):1361–1369. PMID:
18951214.
47. Li L, Tang M, Nie D, Gou J, Li Z. Para-aortic lymphadenectomy did not improve overall survival among women with type I endometrial cancer. Int J Gynaecol Obstet. 2020; 150(2):163–168. PMID:
32433783.
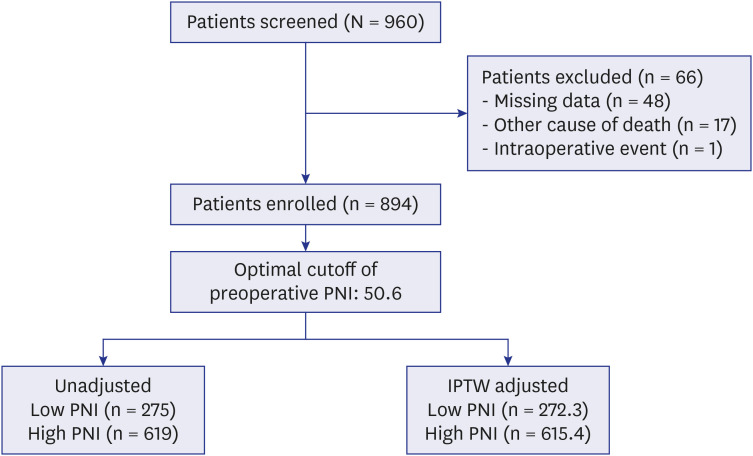
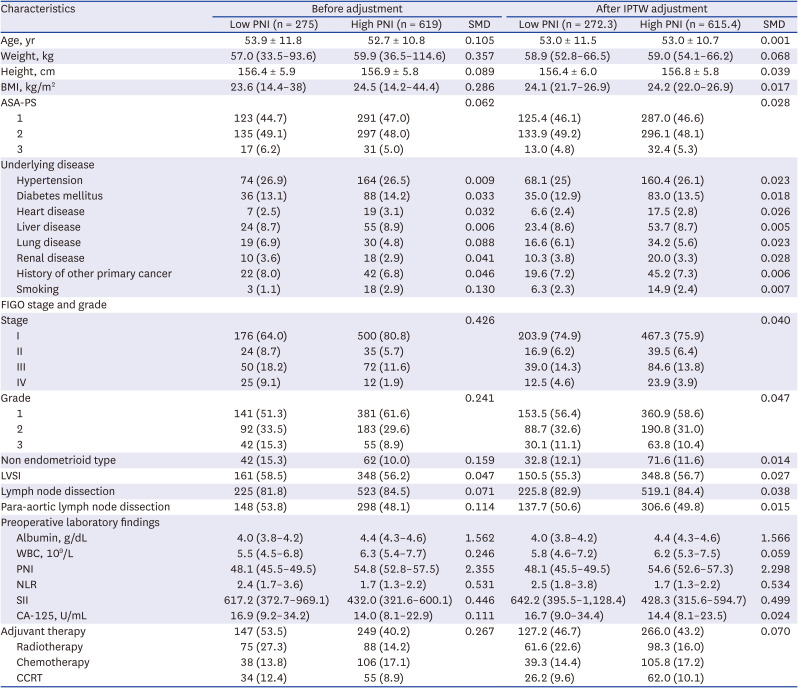
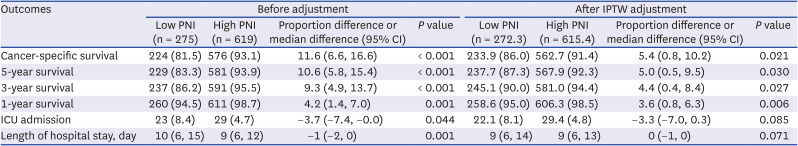
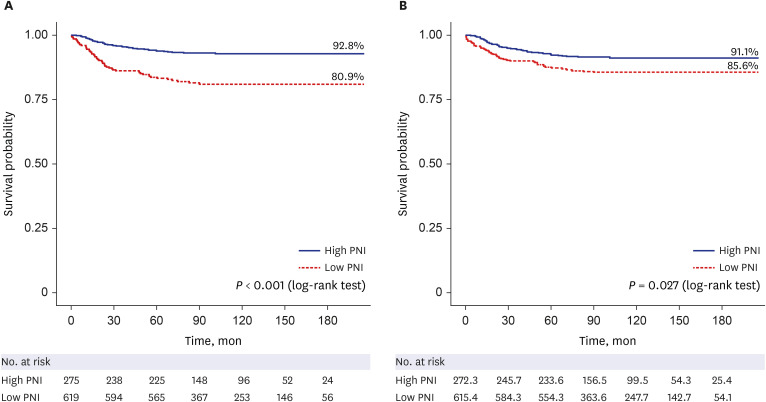




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download