This article has been
cited by other articles in ScienceCentral.
Inflammatory bowel disease (IBD) is a chronic refractory intestinal inflammatory disorder and its pathogenesis is related with several factors including genetic, environmental, microbiota, and immune response, but remains largely unknown. In recent decades, gut microbiota composition and function have become a relevant aspect of IBD pathogenesis and expected promising therapeutic options including probiotics and fecal microbiota transplantation [
1-
3]. Yoon et al. [
4] found a novel commensal
Escherichia coli strain named atypical
E. coli (atEc), which demonstrated anti-inflammatory effect because of 1 additional catalase gene compared to typical
E. coli [
3,
4]. Also, the short-chain fatty acids produced by gut microbiota, such as acetate, propionate, and butyrate are expected to maintain intestinal homeostasis [
5,
6]. We hypothesized to design a novel butyrate-producing recombinant atEc strain using a 2-plasmid system and demonstrate its anti-inflammatory effect in murine colitis model.
A novel recombinant butyrate atEc strain was designed using a 2-plasmid system capable of introducing foreign genes and overexpressing genes for gene expression in the butyric acid metabolic cycle (
Supplementary Fig. 1,
Supplementary Table 1).[
7]. Animals were randomly divided into 4 groups; control+phosphate buffered saline (PBS) (n = 3), dextran sulfate sodium (DSS)+PBS (n = 4), DSS+atEc (n = 4), and DSS+butyrate atEc groups (n = 4). Male C57BL/6 mice (8-week-old) were treated with 1 × 10
8 bacteria in 0.2 mL PBS daily by oral gavage before and after the induction of DSS colitis (from day 0 to day 12) (
Fig. 1A). DSS (2.5% [w/v]; MP Biomedicals, Solon, OH, USA) was added to their drinking water from day 3 of gavage and continued for day 9, and water was changed to DSS-free water before euthanization. All mice were sacrificed 12 days after the onset of the study. Mice were monitored for survival, body weight loss, disease activity index, and colon length. Parts of the distal colon were cut into 2 to 3 pieces for periodic acid-Schiff staining and histologic scoring was obtained. All animal testing complied with all applicable Korean laws and was approved by the Institutional Animal Care and Use Committee of Yonsei University Severance Hospital (Seoul, Korea) (IACUC No. 2019-0241). The experiments complied with the approved IACUC guidelines. All results are expressed as standard error of the mean. GraphPad Software (La Jolla, CA, USA) was used for all analyses. The significance of differences between conditions was assessed using one-way or two-way analysis of variance followed by Holm-Sidak or Tukey’s multiple comparison test, respectively.
P-values < 0.05 were considered significant.
Fig. 1.
Anti-inflammatory effects of butyrate-producing atypical Escherichia coli in murine DSS colitis model. (A) Scheme of the experiment. Eight-week-old male C57BL/6 mice were treated 1×108 bacteria or PBS daily by oral gavage. (B) Body weight was checked during the 12 days before and after induction of colitis by 2.5% DSS added to the drinking water from day 3 of gavage and continued for 7 days. All mice were euthanized on day 12 of the study and disease activity index (C), colon length (D), periodic acid-Schiff staining (E), histopathological score (F), and survival rates (G) were analyzed. Data are expressed as the mean±standard error of the mean (n=4). Analyses were performed using two-way analysis of variance (ANOVA) followed by Tukey multiple comparison test (B, C) and one-way ANOVA followed by Holm-Sidak multiple comparison test (D, F). avs. control: P<0.001, b-dvs. DSS+PBS group or between indicated groups: bP<0.05, cP<0.01, cP<0.001. PBS, phosphate buffered saline-treated vehicle; DSS, dextran sulfate sodium treated; atEc, atypical E. coli-treated; butyrate atEc, butyrate atypical E. coli-treated.
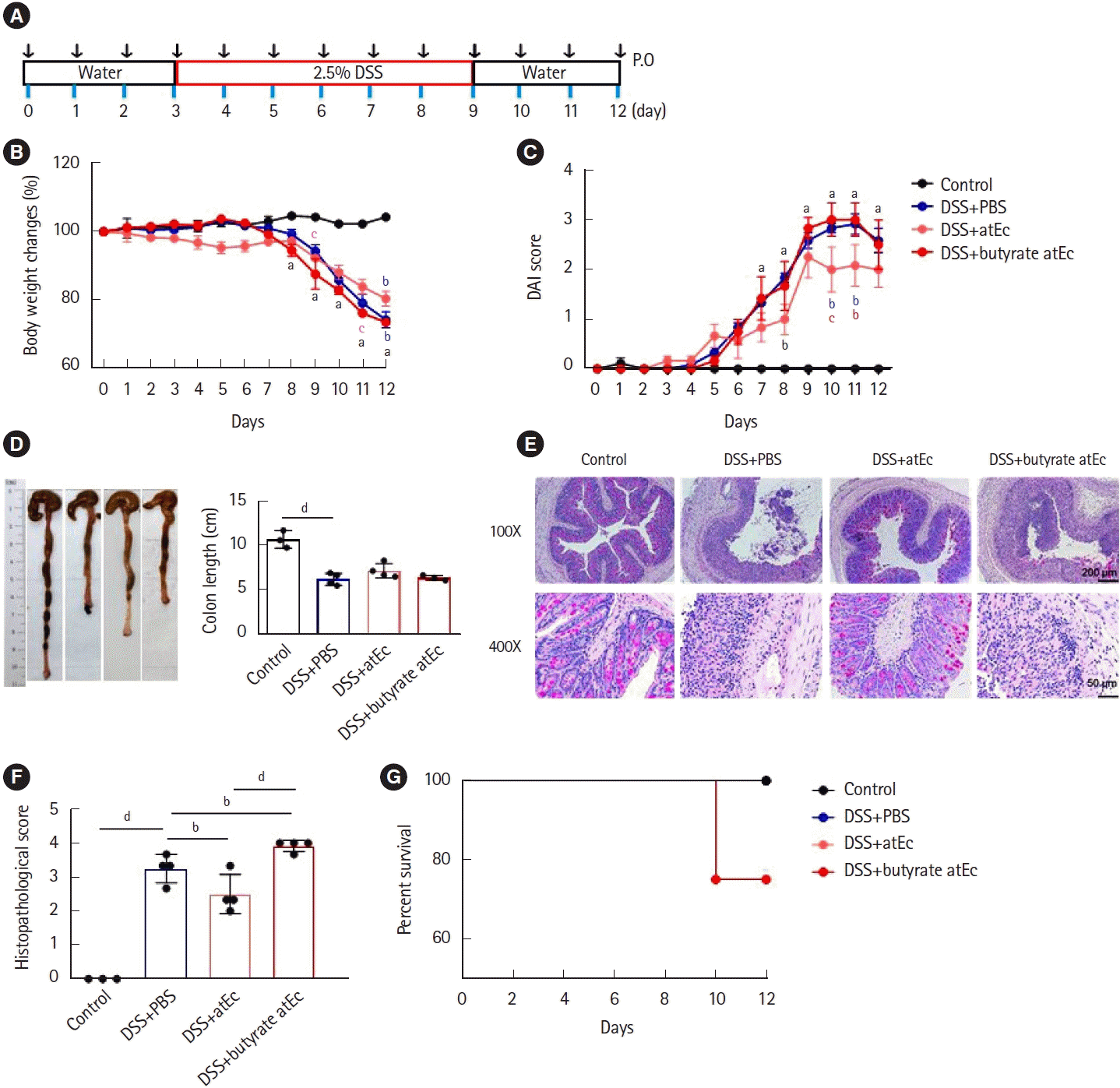

DSS+atEc group attenuated body weight loss (
Fig. 1B), disease activity index (
Fig. 1C), and histopathologic score (
Fig. 1E) compared to DSS+PBS group. However, DSS+butyrate atEc group failed to ameliorate DSS colitis compared to DSS+PBS group (
Fig. 1B-
F). Also, only 1 mouse died after the 10th day in the DSS+butyrate atEc group during the experiment (
Fig. G).
Butyrate-producing bacteria have been expected to have a therapeutic potential in IBD patients to promote intestinal barrier function and modulate immune responses [
8]. Previously, we proved the anti-inflammatory effect of atEc in murine colitis model [
2]. We also developed a novel recombinant butyrate atEc strain using 2 plasmid system and demonstrated its butyrate-producing ability at the cellular level. However, we failed to demonstrate the anti-inflammatory effect of butyrate atEc
in vivo. First, contrary to our hypothesis, butyrate may not only have a good effect, but also have a bad effect on the gut. Zhang et al. [
9] found that colonic short-chain fatty acid in certain chemical microenvironment could activate the growth and motility of pathosymbionts
E. coli strain. Second, although butyrate was well produced by butyrate atEc strain in vitro setting, there is a possibility that it was not well produced in the intestinal environment affected by various confounders. Measurement of luminal butyrate level could be helpful to demonstrate the effect of butyrate at
E. coli strain
in vivo. Further study with butyrate-producing atEc strain with the pretreatment of antibiotics may increase the stable colonization of this bacteria overcoming the normal flora present. Third, a genetically recombined butyrate-producing strain can affect the composition of various metabolites and microbiomes in the gut, which can affect outcomes. Butyrate production efforts with metabolically engineered
E. coli under oxygen-limited conditions had limited success in previous studies [
10]. Therefore, further studies should be warranted to develop a novel strain that works in the intestinal environment.

