3. Miyabe K, Zen Y, Cornell LD, Rajagopalan G, Chowdhary VR, Roberts LR, et al. 2018; Gastrointestinal and extra-intestinal manifestations of IgG4-related disease. Gastroenterology. 155:990–1003.e1. DOI:
10.1053/j.gastro.2018.06.082. PMID:
30012334.

4. Perugino CA, Stone JH. 2020; IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 16:702–14. DOI:
10.1038/s41584-020-0500-7. PMID:
32939060.

6. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. 2012; Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 22:21–30. DOI:
10.3109/s10165-011-0571-z. PMID:
22218969.

7. Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, et al. 2020; The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 79:77–87. DOI:
10.1136/annrheumdis-2019-216561. PMID:
31796497.

8. Ngwa T, Law R, Hart P, Smyrk TC, Chari ST. 2015; Serum IgG4 elevation in pancreatic cancer: diagnostic and prognostic significance and association with autoimmune pancreatitis. Pancreas. 44:557–60. DOI:
10.1097/MPA.0000000000000297. PMID:
25785724.
9. Pan Q, Guo L, Wu J, Cai J, Liao H, Lan Q, et al. 2016; Association between IgG4 autoantibody and complement abnormalities in systemic lupus erythematosus. Mediators Inflamm. 2016:2196986. DOI:
10.1155/2016/2196986. PMID:
27597802. PMCID:
PMC4997081.

10. Takeoka S, Kamata M, Hau CS, Tateishi M, Fukaya S, Hayashi K, et al. 2018; Evaluation of IgG4+ plasma cell infiltration in patients with systemic plasmacytosis and other plasma cell-infiltrating skin diseases. Acta Derm Venereol. 98:506–11. DOI:
10.2340/00015555-2909. PMID:
29437186.

11. Yu KH, Chan TM, Tsai PH, Chen CH, Chang PY. 2015; Diagnostic performance of serum IgG4 levels in patients with IgG4-related disease. Medicine (Baltimore). 94:e1707. DOI:
10.1097/MD.0000000000001707. PMID:
26469909. PMCID:
PMC4616795.

12. Xu WL, Ling YC, Wang ZK, Deng F. 2016; Diagnostic performance of serum IgG4 level for IgG4-related disease: a meta-analysis. Sci Rep. 6:32035. DOI:
10.1038/srep32035. PMID:
27558881. PMCID:
PMC4997323.

13. Usami Y, Ichihara K, Uehara T, Sugano M, Ishimine N, Kawasaki K, et al. 2020; Evaluation of a novel serum IgG4 assay and determination of reference interval for the Japanese population. Clin Chim Acta. 501:136–41. DOI:
10.1016/j.cca.2019.10.032. PMID:
31730813.

14. Culver EL, Sadler R, Simpson D, Cargill T, Makuch M, Bateman AC, et al. 2016; Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol. 111:733–43. DOI:
10.1038/ajg.2016.40. PMID:
27091321.

15. Chibbar R, Wright GR, Dokouhaki P, Dumanski S, Prasad B, Mengel M, et al. 2018; Recurrent IgG4-related tubulointerstitial nephritis concurrent with chronic active antibody mediated rejection: A case report. Am J Transplant. 18:1799–803. DOI:
10.1111/ajt.14758. PMID:
29607610.

18. Moon SH, Kim MH, Lee JK, Baek S, Woo YS, Cho DH, et al. 2017; Development of a scoring system for differentiating IgG4-related sclerosing cholangitis from primary sclerosing cholangitis. J Gastroenterol. 52:483–93. DOI:
10.1007/s00535-016-1246-5. PMID:
27470434.

19. Kamisawa T, Zen Y, Nakazawa T, Okazaki K. 2018; Advances in IgG4-related pancreatobiliary diseases. Lancet Gastroenterol Hepatol. 3:575–85. DOI:
10.1016/S2468-1253(18)30121-3. PMID:
30047448.

20. Lian M, Li B, Xiao X, Yang Y, Jiang P, Yan L, et al. 2017; Comparative clinical characteristics and natural history of three variants of sclerosing cholangitis: IgG4-related SC, PSC/AIH and PSC alone. Autoimmun Rev. 16:875–82. DOI:
10.1016/j.autrev.2017.05.018. PMID:
28564616.

21. Lanzillotta M, Mancuso G, Della-Torre E. 2020; Advances in the diagnosis and management of IgG4 related disease. BMJ. 369:m1067. DOI:
10.1136/bmj.m1067. PMID:
32546500.

22. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. 2015; The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 74:14–8. DOI:
10.1136/annrheumdis-2013-204907. PMID:
24651618.

23. Wang H, Wang C, Wan Q, Li L. 2023; Roles of IgG4 and IgG4/IgG ratio to IgG4-related disease in patients with elevated serum IgG4 level. Clin Rheumatol. 42:793–800. DOI:
10.1007/s10067-022-06413-7. PMID:
36305979.

24. Usami Y, Sugano M, Uehara T, Koinuma M, Ishimine N, Kawasaki K, et al. 2021; Cut-off values of serum IgG4 among three reagents, including a novel IgG4 reagent: a multicenter study. Sci Rep. 11:7280. DOI:
10.1038/s41598-021-86024-5. PMID:
33790306. PMCID:
PMC8012344.

25. Kwon OC, Park MC, Kim YG. 2022; Correlation between serologic parameters and disease activity of IgG4-related disease: differences between patients with normal and elevated serum IgG4 concentrations. Front Immunol. 13:1020459. DOI:
10.3389/fimmu.2022.1020459. PMID:
36311699. PMCID:
PMC9608652.

26. Lin W, Lu S, Chen H, Wu Q, Fei Y, Li M, et al. 2015; Clinical characteristics of immunoglobulin G4-related disease: a prospective study of 118 Chinese patients. Rheumatology (Oxford). 54:1982–90. DOI:
10.1093/rheumatology/kev203. PMID:
26106212.

27. Xia CS, Fan CH, Liu YY. 2017; Diagnostic performances of serum IgG4 concentration and IgG4/IgG ratio in IgG4-related disease. Clin Rheumatol. 36:2769–74. DOI:
10.1007/s10067-017-3685-7. PMID:
28540606.

28. Yamada K, Yamamoto M, Saeki T, Mizushima I, Matsui S, Fujisawa Y, et al. 2017; New clues to the nature of immunoglobulin G4-related disease: a retrospective Japanese multicenter study of baseline clinical features of 334 cases. Arthritis Res Ther. 19:262. DOI:
10.1186/s13075-017-1467-x. PMID:
29191210. PMCID:
PMC5709928.

29. Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, et al. 2015; IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 67:2466–75. DOI:
10.1002/art.39205. PMID:
25988916. PMCID:
PMC4621270.

30. Harkness T, Fu X, Zhang Y, Choi HK, Stone JH, Blumenthal KG, et al. 2020; Immunoglobulin G and immunoglobulin G subclass concentrations differ according to sex and race. Ann Allergy Asthma Immunol. 125:190–5.e2. DOI:
10.1016/j.anai.2020.03.018. PMID:
32224206. PMCID:
PMC7387162.

31. Puissant-Lubrano B, Peres M, Apoil PA, Congy-Jolivet N, Roubinet F, Blancher A. 2015; Immunoglobulin IgA, IgD, IgG, IgM and IgG subclass reference values in adults. Clin Chem Lab Med. 53:e359–61. DOI:
10.1515/cclm-2014-1186. PMID:
25720098.

32. Li P, Liu Z, Wu Z, Wen X, Li L, Zhang S, et al. 2017; Adult reference intervals for IgG subclasses with Siemens immunonephelometric assays in Chinese population. Allergy Asthma Clin Immunol. 13:44. DOI:
10.1186/s13223-017-0216-7. PMID:
29042863. PMCID:
PMC5629794.

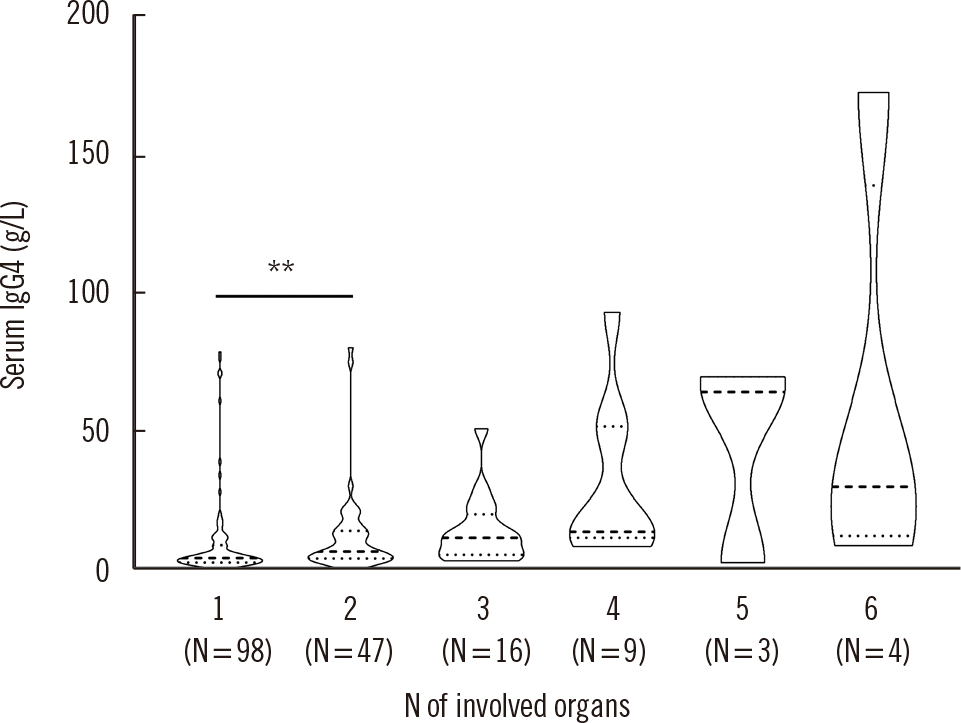
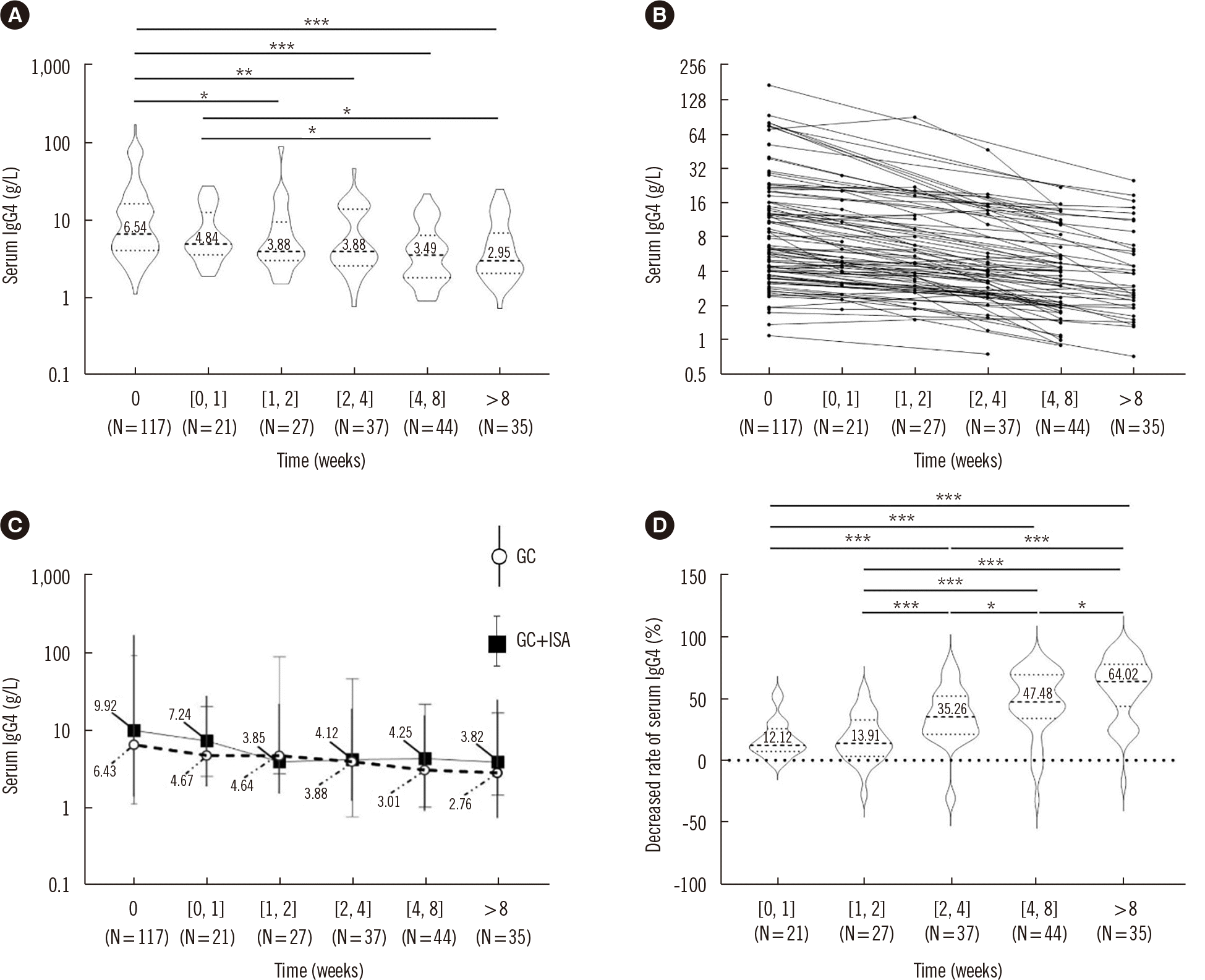




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download