1. Park HY, Shin JH, Boo HO, Gorinstein S, Ahn YG. Discrimination of
Platycodon grandiflorum and
Codonopsis lanceolata using gas chromatography-mass spectrometry-based metabolomics approach. Talanta. 2019; 192:486–491. PMID:
30348422.
2. Zhang L, Wang Y, Yang D, Zhang C, Zhang N, Li M, Liu Y.
Platycodon grandiflorus - an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2015; 164:147–161. PMID:
25666431.
3. Nyakudya E, Jeong JH, Lee NK, Jeong YS. Platycosides from the roots of
Platycodon grandiflorum and their health benefits. Prev Nutr Food Sci. 2014; 19:59–68. PMID:
25054103.
4. Ji MY, Bo A, Yang M, Xu JF, Jiang LL, Zhou BC, Li MH. The pharmacological effects and health benefits of Platycodon grandiflorus - a medicine food homology species. Foods. 2020; 9:E142.
5. Li W, Tian YH, Liu Y, Wang Z, Tang S, Zhang J, Wang YP. Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J Toxicol Sci. 2016; 41:417–428. PMID:
27193733.
6. Huang YH, Jung DW, Lee OH, Kang IJ. Fermented
Platycodon grandiflorum extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mice. J Med Food. 2016; 19:1004–1014. PMID:
27792464.
7. Jung JI, Lee HS, Kim SM, Kim S, Lim J, Woo M, Kim EJ. Immunostimulatory activity of hydrolyzed and fermented Platycodon grandiflorum extract occurs via the MAPK and NF-κB signaling pathway in RAW 264.7 cells. Nutr Res Pract. 2022; 16:e39.
8. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001; 357:1777–1789. PMID:
11403834.
9. Wang K, Conlon M, Ren W, Chen BB, Bączek T. Natural products as targeted modulators of the immune system. J Immunol Res. 2018; 2018:7862782. PMID:
30809554.
10. Sakthivel KM, Guruvayoorappan C.
Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice. J Immunotoxicol. 2015; 12:154–163. PMID:
24873678.
11. Pass GJ, Carrie D, Boylan M, Lorimore S, Wright E, Houston B, Henderson CJ, Wolf CR. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005; 65:4211–4217. PMID:
15899812.
12. Tripathi DN, Jena GB. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: a study in mice. Chem Biol Interact. 2009; 180:398–406. PMID:
19539803.
13. Noh EM, Kim JM, Lee HY, Song HK, Joung SO, Yang HJ, Kim MJ, Kim KS, Lee YR. Immuno-enhancement effects of
Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement Altern Med. 2019; 19:322. PMID:
31752816.
14. Zhou Y, Chen X, Yi R, Li G, Sun P, Qian Y, Zhao X. Immunomodulatory effect of tremella polysaccharides against cyclophosphamide-induced immunosuppression in mice. Molecules. 2018; 23:E239.
15. Yu Q, Nie SP, Wang JQ, Liu XZ, Yin PF, Huang DF, Li WJ, Gong DM, Xie MY. Chemoprotective effects of
Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. Int J Biol Macromol. 2014; 64:395–401. PMID:
24370474.
16. Lee S, Han EH, Lim MK, Lee SH, Yu HJ, Lim YH, Kang S. Fermented
Platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity
in vivo. J Med Food. 2020; 23:1060–1069. PMID:
32758004.
17. Wu Y, Zhu CP, Zhang Y, Li Y, Sun JR. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int J Biol Macromol. 2019; 137:504–511. PMID:
31229542.
18. Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A. 2010; 107:19985–19990. PMID:
21045130.
19. Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006; 208:270–282. PMID:
16362985.
20. Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, Winslow GM. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. 2011; 186:1011–1021. PMID:
21148037.
21. Wang J, Tong X, Li P, Cao H, Su W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in BALB/c mice. J Ethnopharmacol. 2012; 139:788–795. PMID:
22212503.
22. Lee I, Kwon DH, Lee SH, Lee SD, Kim DW, Lee JH, Hyun SK, Kang KH, Kim CM, Kim BW, et al. Immuno-modulation effect of Ulmus macrocarpa Hance water extract on BALB/c mice. J Life Sci. 2014; 24:1151–1156.
23. Ryu HS. Effects of water extract from Platycodon grandiflorum on mouse immune cell activation ex vivo by oral administration. Korean J Food Nutr. 2014; 27:99–104.
24. Ader JL, Rostaing L. Cyclosporin nephrotoxicity: pathophysiology and comparison with FK-506. Curr Opin Nephrol Hypertens. 1998; 7:539–545. PMID:
9818201.
25. Artym J, Zimecki M, Kruzel ML. Effect of lactoferrin on the methotrexate-induced suppression of the cellular and humoral immune response in mice. Anticancer Res. 2004; 24:3831–3836. PMID:
15736418.
26. Kim HS, Chung KT, Lee IH, Choi WB, Lee JH, Hyun SK, Kim BW, Hwang HJ. Effect of Alpina officinarum ethanol extract on immunoregulatory activities in the mice. J Life Sci. 2014; 24:61–66.
27. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004; 351:2715–2729. PMID:
15616206.
28. Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013; 39:806–818. PMID:
24238338.
29. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001; 17:387–403. PMID:
11687494.
30. Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007; 131:994–1008. PMID:
18045540.
31. Lee EB, Lee SH, Park YG, Choi JH, Lee HK, Jang HH, Hwang GA, Choe JS, Park SY, Choi AJ, et al.
Platycodon grandiflorum extract ameliorates cyclophosphamide-induced immunosuppression in mice. J East Asian Soc Diet Life. 2019; 29:303–309.
32. Jung CW, Kim SJ, Anh NH, Lee EG, Kim TH, Kwon SW, et al. The clinical effects of Platycodon grandiflorum: a systematic review. Korean J Pharmacogn. 2021; 52:1–12.
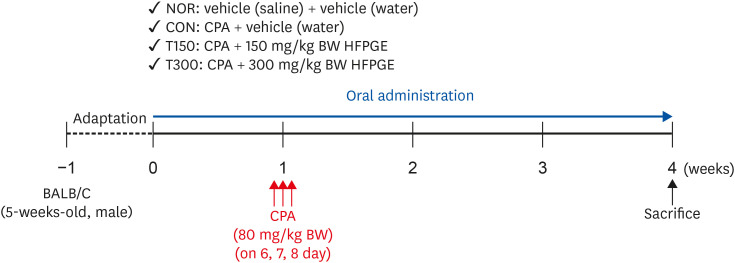
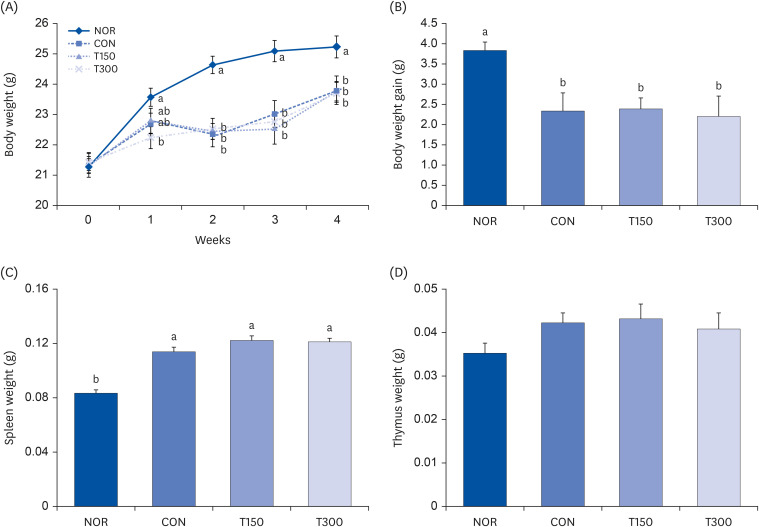
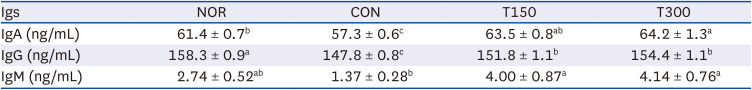
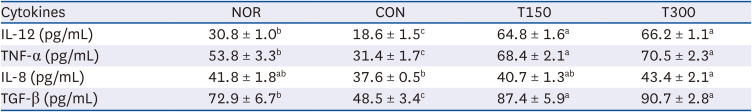





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download